Translate this page into:
The Use of Methadone in Adult Patients with Cancer Pain at a Governmental Cancer Center in India
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Management of cancer-related pain relies on the access to opioids. When regular opioids as morphine are not tolerated or are insufficient, adjuvant opioids as methadone are an affordable and effective analgesic.
Aim:
The aim of the project was to describe the pattern of use and clinical experiences of methadone in patients with cancer-related pain at a low-resource hospital in Hyderabad, one of few Indian cancer centers with permission to prescribe methadone.
Methods:
Medical records of all patients who had been prescribed methadone, September 9, 2017 and November 19, 2019 were studied retrospectively. Data on analgesic treatment and opioid side effects were analyzed.
Results:
A total of 93 adult cancer patients were included in the study. A majority of patients (79%) were prescribed opioid analgesic, mainly morphine, before methadone introduction. The initial daily dose of methadone ranged between 5 and 22.5 years and in the vast majority of the patients 5 mg, divided in two daily administrations. A good analgesic effect, with decreased pain, was reported in 60% of the patients. No severe side effects were reported.
Conclusions:
In this study, methadone as a primary opioid was used with a good analgesic effect for cancer pain in a low-resource setting. Indication for methadone was mainly uncontrolled pain with a regular opioid treatment. No severe adverse effects were reported. Further research and prospective studies are needed on methadone treatment in low-resource settings to establish the robust guidelines to support prescribing physicians.
Keywords
Cancer
methadone
opioid
pain
palliative care
INTRODUCTION
Background
Treatment of cancer-related pain, defined as pain caused either by the disease itself or by the treatment of the disease, is an important part of the supportive care for all cancer patients. The World Health Organization (WHO) estimates that the global cancer incidence will rise from the current number of 18.1 million new cases each year to a total of 29.5 million new cases in the year of 2040.[12] In India, a low-middle income country (LMIC), the Global Cancer Observatory reported approximately 1.2 million new cases of cancer in 2018.
Cancer pain is burdensome and has a large impact on many aspects of the patient's life. Under-treatment of cancer pain may increase the risk for depression and impair patients' coping strategies for the various troublesome symptoms that occur.[34]
Cancer-related pain is caused by pressure from the tumor on adjacent organs/tissues or by the tumor invading the tissue and damaging it. The main types of pain are often described as nociceptive and neuropathic pain, respectively. Nociceptive pain arises from nociceptors located throughout our body, which mediate the signals to notify us of a potentially harmful stimulus.[5] Neuropathic cancer pain is associated with a significantly worsened quality of life and correlates with an aggravated pain.[6] The prevalence of neuropathic pain among cancer patients is reported to be 40%.[7]
Treatment of malignant pain requires a combination of different medications. Opioids are the backbone of the pharmacologic treatment. The WHO analgesic ladder, for relief of cancer-pain in adults, was published in 1996[8] and presented a model of hierarchy in analgesic therapy. In the ladder, paracetamol and nonsteroidal anti-inflammatory drugs are represented in the first step followed by mild and strong opioids in the second and third steps, respectively. Recently, a working group for cancer pain management in low-resource settings (the CAPER working group) proposed a different strategy to make analgesic therapy more manageable in the low-resource setting, i.e., in LMIC. They suggest in their algorithm the introduction of short-acting opioids for opioid naïve patients already when pain-assessment is reported to be above three on a numerical rating scale (NRS) where 0 is freedom from pain and 10 is the worst possible pain.[9] The WHO has listed opioids as essential medicines, i.e., medicines for worldwide priority health-care needs; however, the access to opioids is very unequally distributed globally.[10] It was estimated, in 2010, regarding legal global opioid consumption in the treatment of pain that four high-income countries (HIC) accounted for 68% of the worldwide consumption, while all lower-middle income countries (LMIC) together only accounted for 7% of the global use.[111213] Furthermore, it is estimated that 83% of the worlds' population is left without effective pain treatment.[111213] This is despite improved knowledge in the field and actions taken by different organizations, among them the WHO, to relieve the global burden of cancer pain.[1114]
Methadone
Methadone is an opioid that was initially developed in the 1940s, as an alternative to morphine. It has been widely used to curb heroin-addiction but has in the recent decade received more attention as an analgesic for neuropathic cancer pain. In clinical pain management, methadone is useful in the treatment of cancer-related pain and has been shown to share similar analgesic benefits as morphine.[15] It is regarded as a long-acting opioid, with an oral bioavailability of 80% (with a range of 41%–99%), which is threefold the bioavailability of oral morphine.[16] The side effects of methadone are to a great extent equal to those of morphine; including nausea, constipation, and drowsiness.[17] A Cochrane review reported methadone to be efficient in neuropathic pain management and a cost-efficient option in many economies, but also that the evidence are yet scarce due to a limited amount of studies.[18] Its pharmacokinetic features are characterized by its affinity to the mu receptor and an antagonistic effect to the N-methyl-d-aspartate-receptor. The challenges with methadone include long half-life with highly pharmacokinetics individual variations of 8.5–47 h;[19] hence, high risk for accumulation that may lead to the development of delayed toxicity, possible drug interactions,[20] concerns over dose titration and conversion from other opioids, and a dose-dependent prolongation on the QT-interval[21] and as a consequence a potential of serious arrhythmias.[2223] In a recent study by Lovell et al. from 2019, a clinically significant difference between the incidence of QT-prolongation was seen between patients treated with low-dose methadone (mean daily dose of 14.3 mg) and patients treated with high-dose methadone (mean daily dose of 86 mg) with an increased risk following the high doses.[24] Patients with baseline QT-prolongation had a higher risk of developing QT-prolongation after 2 weeks of treatment compared to patients without a baseline QT-prolongation.[25]
Methadone in India
The prescription of methadone is legal since 2014.[26] Today, any hospital can apply for a license to procure methadone but up until now only a few centers have a license, whereof at the study hospital since September 2017. A hospital has to go through a license process to achieve a Recognized Medical Institution (RMI)–status, which entails several steps to ensure a safe use of methadone. A hospital is required to have sufficient facilities to see patients, a facility to safely store methadone and trained recognized medical practitioners, for the usage of and prescription of opioids. The drug control authority (DCA) then issues a certificate of RMI. The hospital then makes a request to DCA for an estimated annual requirement of methadone prescription.
However, methadone is still afflicted by distrust and misconceptions, hence there is a need for a better understanding, and a safe introduction, of methadone in India, as well as in other low-resource settings[27] in LMICs over the world.
Aim
The aim of the project was to describe the pattern of use and clinical experiences of methadone in patients with cancer-related pain at a low-resource hospital in India.
METHODS
Study design
The study was implemented as a descriptive retrospective study in adult cancer patients who had previously been, or were currently, prescribed methadone at Mehdi Nawaz Jung Institute of Oncology and Regional Cancer Center (MNJIO).
At MNJIO, methadone is prescribed only from the Department of Pain and Palliative Care (DPPC). The patient collects the prescribed amount of methadone, available as tablets (5 mg) or oral suspension (5 mg/ml) from the hospital pharmacy.
Patient selection
Consecutive data of all adult (>18-year-old) cancer patients receiving methadone at MNJIO, from the beginning of the methadone permission period, from September 9th, 2017 until November 19th, 2019 were collected. Patients were identified through a separate register of methadone-prescriptions kept by the local staff at the department of palliative care.
Data collection and parameters
Patient-specific information as gender, age, distance to the hospital, care-giving location (home care, hospital, hospice, or the three in combination), and socioeconomic status were collected. Furthermore, cancer diagnosis and on-going curative or palliative tumor-specific treatment were documented.
Type of pain was classified as nociceptive, neuropathic, or mixed pain. Indication for methadone treatment was recorded. Documentation of investigations before the initiation of methadone including recent (not older than two months) blood tests of sodium, potassium, creatinine and albumin, and electrocardiogram (ECG) (not older than six months) were registered.
Analgesic pharmacological treatment preceding methadone initiation was recorded, and the Morphine Equivalent Daily Doses (MEDD) were calculated according to data provided by Center for Disease Control and Prevention for all opioids. Concomitant analgesic treatment in conjunction with opioids was listed.
Records of the analgesic effect of methadone were searched for in the medical records. Pain-scores, with the NRS for pain,[28] were sought for before and after methadone initiation. In case of missing data an assumption of pain as “pain relief” (NRS 0), “mild pain” (NRS 1–3), “pain” (NRS 4–6) or “severe pain” (NRS 7–10) was recorded. Furthermore, NRS scores found in records were translated into these categories, i.e., “mild pain” (NRS 1–3), “pain” (NRS 4–6), or “severe pain” (NRS 7–10) was then recorded. When no NRS rating was performed, the presence of the word “pain-relief” in records was assumed to equal NRS 0 and noted.
Notes on side effects were sought for in the medical records. The presence of the words “jerky movements,” “confusion,” “irrelevant talk,” or “delirium” was noted as delirium in the study protocol.
Reasons for discontinuation of methadone were documented, as side effects, abandonment from treatment, death, or other reasons.
All data were collected from the patients' medical records at the hospital. In a few cases, additional data were collected from the hospice and from the home care files, respectively.
Statistical analysis
Descriptive analysis was used to summarize the demographic data. The obtained data were not symmetrical distributed. Due to the large variety in the results, the median was used as the measure of central tendency. Data calculation was performed with Microsoft Corporation, Excel 2016 (v16.0), Redmond, Washington, US.
Ethics approval
Ethics approval by the ethical board at the study hospital was obtained prior to initiation of the study. The data were compiled in an anonymous manner, and the patients could therefore not be identified.
RESULTS
A total number of 115 adult cancer patients had been prescribed methadone at MNJIO cancer hospital between September 9, 2017 and November 19, 2019, according to the register kept at the DPPC. Of these, 16 records could not be found, and additional six records did not contain any information about methadone and were thus excluded. The study population hence consisted of 93 patients.
Patient characteristics
The median age was 45 (19–77) years in the 61 males and 32 females included in the analysis. A majority of the patients, 75% (70/93), lived below the poverty threshold[29] (annual family income below 200 000 INR) and 31% (29/93) lived more than 50 kilometers from the hospital. Cancer diagnoses were dominated by head-and-neck cancer, followed by gynecological, gastrointestinal, lung, and breast cancer. As methadone-treatment was introduced, 47% (44/93) of the patients were currently on tumor-specific treatment, whereof 52% (23/44) were treated with a curative intent. Patient-specific data are shown in Table 1.
| n (%) | |
|---|---|
| Gender | |
| Female | 32/93 (34) |
| Male | 61/93 (66) |
| Age (years) – median (range) | 45 (19–77) |
| Socioeconomic status | |
| White card holder* | 70/93 (75) |
| All other types | 23/93 (25) |
| Distance to hospital (km) | |
| <50 | 64/93 (69) |
| >50 | 29/93 (31) |
| Cancer types | |
| Head-neck cancer | 60/93 (64.5) |
| Gynecological | 11/93 (11.8) |
| Gastrointestinal | 6/93 (6.4) |
| Urologic | 6/93 (6.4) |
| Lung | 3/93 (3.2) |
| Breast | 1/93 (1,0) |
| Other | 6/93 (6.4) |
| Tumor-specific treatment | |
| Yes | 44/93 (47) |
| No | 49/93 (53) |
| Intention of tumor-specific treatment | |
| Palliative | 21/44 (48) |
| Curative | 23/44 (52) |
Pain and indication for methadone treatment
Indication for methadone was mainly increased or unresolved pain on current analgesic treatment 74% (69/93) and in a few cases, 4% (4/93), due to side-effects from existing treatment with regular opioids. Indication for initiation of methadone was classified as “other” in 5% (5/93) patients. This could be due to a stock of methadone tablets at the hospital pharmacy with a short expiring date, and physicians were therefore asked to prescribe methadone. In 16% (15/93) of the patients' records, there was no explicit mentioning on the cause for initiating methadone.
Pain, at the time of methadone introduction, was classified as mixed in 79% (73/93) of the patients, in 19% (18/93) as neuropathic pain, and information was missing in 2% (2/93) patients.
Baseline investigations
In 28% (26/93) of the patients' ECG was recorded before the introduction of methadone. Blood samples for sodium and potassium were registered in 35% (33/93) and creatinine and albumin were assessed in 60% (56/93) of patients. An increased value of creatinine, above the range for normal kidney function, was found in 5% (5/93) patients.
Primary opioids prior to methadone introduction
Prior to methadone introduction, 79% (73/93) of the patients were prescribed opioid analgesics and 4% (4/93) patients were prescribed nonopioid analgesics. Information regarding analgesics before methadone introduction was missing in 17% (16/93) patients. Morphine was the most common primary opioid before methadone introduction, in 68% (50/73). Second to morphine was tramadol, in 26% (19/73) of patients and thirdly fentanyl in 5% (4/73) of patients. In the 50 patients on morphine 20 received morphine alone, in the remaining 30 patients in different combinations with valproate, paracetamol, gabapentin, tramadol, fentanyl, or amitriptyline.
The median MEDD before the introduction of methadone treatment was 30 (10–420) mg with in a single outlier 1000 mg, with the exception of seven patients where the data on opioid doses were missing.
Methadone treatment
Methadone replaced the existing primary opioid in 79% (73/93) of the patients, i.e., an opioid rotation was performed, or, as in the 4% (4/93) opioid-naive patients it was introduced as the first-line opioid. Methadone was never used as an adjuvant opioid, i.e., as an add-on treatment to an existing primary opioid. Methadone was introduced at the hospital in 85% (79/93) of the patients, and at the hospice, or by a home-care team, in 10% (9/93) and 5% (5/93) of the patients, respectively.
Dosing and titration of methadone
The initial daily dose of methadone ranged between 5 and 22.5 mg, in the vast majority (60/93 patients) 5 mg was the starting dose. Number of daily administrations was once (5/93), twice (75/93) or three (13/93) times daily. Approximately half of the patients were prescribed tables 53% (49/93), and the rest were prescribed oral suspension 47% (44/93).
In the study sample, 21% (20/93) of patients were prescribed methadone once (one single prescription), equaling 1–2 weeks of treatment. In the 79% (73/93) of patients where methadone had been prescribed more than once a dose-escalation was commonly made with the addition of 5 mg to a final median dose of 7.5 mg after three prescriptions [Figure 2]. From the first to the very last prescription, methadone doses increased in 60% (44/73), unchanged in 37% (27/73) and reduced in 3% (2/73) of patients. The time interval between prescriptions iteration and number of prescriptions iterated varied widely between patients.
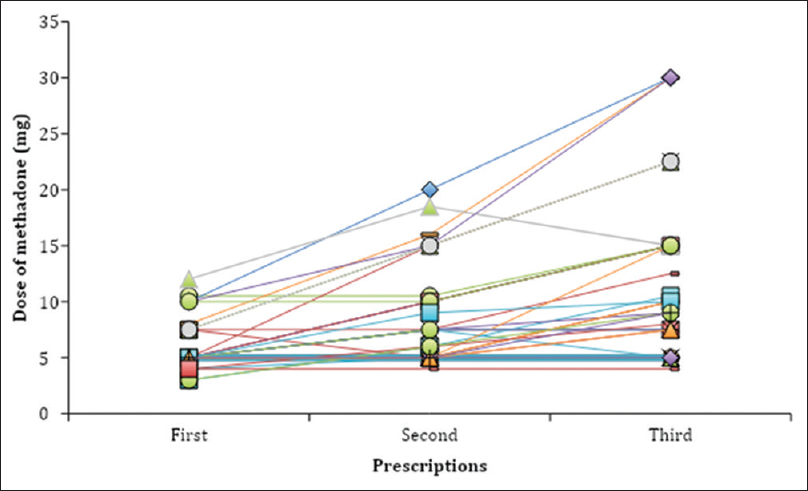
- Dose titration of methadone during the first three prescriptions of methadone, both tablet and oral suspension (mg). Each line represents one patient. The time interval between the first, second, and third prescription varied widely
Duration of methadone treatment
In 18 of the 93 patients,' data on duration on treatment were missing.
Patients (26/93) who continued and remained on methadone on the last date of observation (n = 9) or until death (n = 17) had a median treatment duration time of 61 (4–398) days. All deaths were disease related. For duration of treatment among patients with more than one prescription see Figure 1.
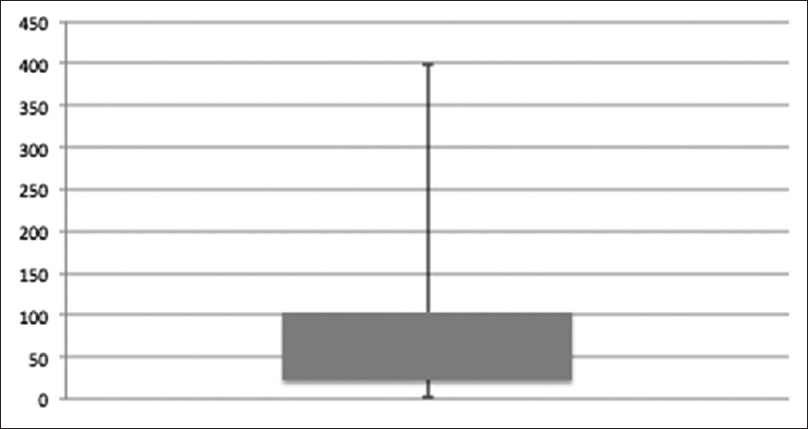
- Box plot describing the number of days on methadone treatment among patients who received methadone prescriptions more than once (73/93). Duration was median 49 (2–398) days
In 37% (35/93) of patients whose methadone treatment was discontinued, the median treatment duration was 45 (2–278) days. Reason for discontinuation was opioid rotation, back to a regular opioid in 80% (39/49) or a switch to nonopioid drugs 2% (1/49), side-effects from methadone 8% (4/49), patient abandonment 6% (3/49), and in 4% (2/49) resolved pain without further need for analgesics.
Analgesic effect of methadone
Pain assessment registration with NRS was found in 68% (63/93) of the patients × records. In the remaining 32% (30/93) no pain assessment, following the introduction of methadone, could be found in the medical records and no assumption of analgesic effect could be done either. Methadone treatment resulted in decreased pain in 60% (38/63), increased pain in 13% (8/63), and pain was unchanged in 27% (17/63) of the patients as measured with NRS [Figure 3].
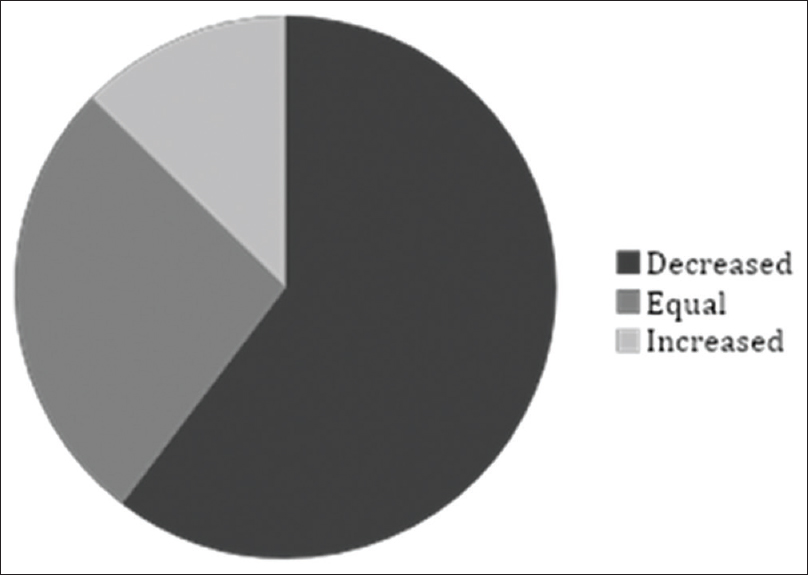
- Patients' pain experience from the first to the last assessment of methadone treatment. Pain was assessed from patients' records according to “pain relief” (numerical rating scale 0), “mild pain” (numerical rating scale 1–3), “pain” (numerical rating scale 4-6) and “severe pain” (numerical rating scale 7–10). Information regarding assessment was missing in 30/93 patients. Patients' experience of pain, defined as reduction in the pain estimation instrument, decreased in 38/63. In 8/63 of patients, the pain increased and 17/63 did not feel any change
(WHO/Eastern Cooperative Oncology Group (ECOG) and Edmonton Symptom Assessment System (ESAS)-scores were present in 32% (30/93) and Palliative Performance Scale (PPS)-scores in 30% (28/93) of the medical records. No analyses can be made due to the large amount of missing data.
Side effects
In 81% (75/93) patients, there were no documented side effects. Side effects, of any kind, were reported in only 19% (18/93) of the patients, whereof nausea occurred in 17% (16/93). Delirium was documented in 9% (8/93) and was reported as “delirious episodes,” with a median of one (1–14) episode per patient. As delirious episodes were noted, the median daily methadone dose was 10 (3–22.5) mg. The methadone treatment was continued in all patients despite episodes of delirium. Some patients suffered both nausea and delirium.
DISCUSSION
In this study, we found that low doses of methadone as a primary opioid, replacing morphine, was safely introduced in the treatment of cancer-related pain, in a low-resource LMIC cancer hospital. This is in contrast with a the prevailing model in HIC, where low-dose methadone mainly is an adjuvant opioid when regular opioid treatment is insufficient, predominantly in pain with neuropathic features, even if there is not yet much evidence for such a strategy.[183031]
In LMIC, as India, where the study was performed, there is a limited access to opioids in pain-treatment, outside larger hospitals, and special permissions are required for the subscription of morphine. Methadone is up until now only available at a few hospitals in India.
Because of long-half life with only a few daily administrations, the high grade of bioavailability and low costs methadone is of particular interest in LMIC.[15] On the backside are the distrusts surrounding methadone. Due to individual variations in pharmacokinetics, there is a risk for accumulation and severe cardiac side effects with QT-prolongation. Methadone is considered to be difficult to titrate with its unique pharmacokinetic features and requires clinical experience and knowledge in assessing and balancing potential risks against the benefits of methadone. Robust guidelines on initial doses, titration schedules, monitoring and management of risks, with the core goal of promoting patient safety and to provide science-based care and security for prescribing physicians are therefore in great need.[20] In the present study, before the introduction of methadone, baseline ECG was controlled only in a minority of patients. Even though ECG was not regularly followed up during methadone treatment, no reports of cardiac events were seen. In the literature, the use of ECG to identify persons at greater risk for methadone associated arrhythmia and follow-up with ECG is recommended during methadone-treatment,[20] but, in the use of low-dose methadone (<30 mg daily) cardiac adverse effects are uncommon and therefore the necessity for ECG monitoring might be questioned. In the use of high-dose methadone (>100 mg daily doses) ECG has an undisputed role and dose-changes should be monitored cautiously.[31]
Limitations to our report are the retrospective nature of this study. Retrieving data revealed missing information both on analgesic effects and side-effects. A number of patients in the present study had only been prescribed methadone once, and in these patients we cannot fully analyze the effects of methadone. Also, the last patients included were followed only a short time.
Albeit this, we trust that serious side effects had been reported had they been noticed. Assumptions on effects and side-effects had to be made when notations in the medical records were scarce. However, the lack of negative remarks and the on-going use of methadone is as a support for appreciation of the drug as safe and effective, among the medical professionals. Estimations of ESAS-scores, WHO/ECOG-scores and PPS-scores were only made in a few records on a small number of occasions. They were thus not comparable and did not contribute to the result of the study.
Prescriptions of methadone were handed to the patient or patients' caregivers to last one and not more than 2 weeks, thus allowing a close monitoring of the patient responsiveness to and side-effects of methadone. In most cases the caregiver was responsible for retrieving the drug and dispensing it to the patients. We registered the prescriptions of methadone from the hospital clinic, daily notes from hospice, and records from the home care facility. This may lead to and could have contributed to some uncertainties in the number of days of methadone treatment. As always, patients' compliance to the prescribed medications is hard to assess.
This study shows, notwithstanding, at a governmental cancer center with limited resources and with patients from the lower socioeconomic strata that low-dose methadone can be a safe, tolerable, and cost-effective primary opioid in the treatment of cancer-related pain.
In concordance with international studies, we could find that methadone was well tolerated with few side effects and in the majority of cases a pain-relief was seen, following the introduction of methadone.[1830] This is of special interest as methadone in the present study was used as a primary opioid, mainly by an opioid-rotation from morphine or as a “de novo” opioid, and not as an adjuvant opioid, as opposed to the most comment usage of methadone in comparable patient groups in HIC. There may be several advantages of this approach in a LMIC setting, in the simplicity, with few daily doses and low costs. With a high patient flow, at a low-resource centers, a low-cost and efficient analgesic therapy is of need for the many under-privileged patients. Methadone could be such an opioid option.
A general use of low-dose methadone as an analgesic in cancer-related pain can be supported provided that methadone is handled with caution and attention, with guidelines customized for a low-resource setting, as illiteracy and disbeliefs might hamper the use of an efficient and affordable treatment.
This study can hopefully contribute to a continued research of methadone in the treatment of malignant pain, thus making efficient analgesic treatment with opioids more equally accessible globally.
CONCLUSIONS
In this study, methadone as a primary opioid has been used for cancer pain in a low-resource setting. Indication for methadone was mainly uncontrolled pain with a regular opioid treatment. Treatment with methadone resulted in no severe adverse effects.
Further research and prospective studies are needed on methadone treatment in low-resource settings to establish robust guidelines to support prescribing physicians.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We wish to thank Mehdi Nawaz Jung Institute of oncology and regional cancer center and the Department of Pain and Palliative Care for providing resources and records for this study. Furthermore, thanks to the Two Worlds Cancer Collaboration (TWCC), Vancouver, Canada, and Pain Relief and Palliative Care Society (PRPCS), Hyderabad, India, for providing resources and support and the Swedish International Development Cooperation Agency for help financing the project.
REFERENCES
- World Health Organization. Cancer Tomorrow. Lyon: International Agency for Research on Cancer. World Health Organization. Available from: URL: https://gco.iarc.fr/tomorrow/home
- [Google Scholar]
- Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-53.
- [Google Scholar]
- Cancer pain and psychosocial factors: A critical review of the literature. J Pain Symptom Manage. 2002;24:526-42.
- [Google Scholar]
- Multicenter, cross-sectional observational study of the impact of neuropathic pain on quality of life in cancer patients. Support Care Cancer. 2017;25:3759-67.
- [Google Scholar]
- Neuropathic pain components in patients with cancer: Prevalence, treatment, and interference with daily activities. Pain Pract. 2016;16:413-21.
- [Google Scholar]
- WHO Library Cataloguing in Publication Data (2nd ed). Geneva: World Health Organization; 1996. p. :145. ISBM 92 4 15 44 8 21 (NLM Classification QZ 200).
- Optimizing cancer pain management in resource-limited settings. Support Care Cancer. 2019;27:2113-24.
- [Google Scholar]
- World Health Organization. 2019. Model List of Essential Medicines. Geneva: World Health Organization; Available from: from: URL: https://apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-201906-engpdfua=1
- [Google Scholar]
- Global Access to Pain Relief Evidence for Action. European Society for Medical Oncology (ESMO). Available from: https://www.esmo.org/content/download/14123/252826/file/Global-Access-to%E2%80%91Pain-Relief-Evidence-for-Action.pdf
- [Google Scholar]
- United Nations. 2006. Report of International Narcotics Control Board for 2004. New York: United Nations; Available from: https://www.incb.org/documents/Publications/AnnualReports/AR2005/AR_05_ English.pdf
- [Google Scholar]
- Availability and utilization of opioids for pain management: Global issues. Ochsner J. 2014;14:208-15.
- [Google Scholar]
- A first comparison between the consumption of and the need for opioid analgesics at country, regional, and global levels. J Pain Palliat Care Pharmacother. 2011;25:6-18.
- [Google Scholar]
- Cross-sectional pilot study to monitor the availability, dispensed prices, and affordability of opioids around the globe. J Pain Symptom Manage. 2014;48:649-590.
- [Google Scholar]
- Methadone for relief of cancer pain: A review of pharmacokinetics, pharmacodynamics, drug interactions and protocols of administration. Support Care Cancer. 2001;9:73-83.
- [Google Scholar]
- Management of opioid side effects in cancer-related and chronic noncancer pain: A systematic review. J Pain. 2003;4:231-56.
- [Google Scholar]
- Methadone-metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551-9.
- [Google Scholar]
- Methadone safety: A clinical practice guideline from the American Pain Society and College on Problems of Drug Dependence, in collaboration with the Heart Rhythm Society. J Pain. 2014;15:321-37.
- [Google Scholar]
- QTc interval prolongation associated with intravenous methadone. Pain. 2003;105:499-506.
- [Google Scholar]
- Methadone-associated Q-T interval prolongation and torsades de pointes. Am J Health Syst Pharm. 2009;66:825-33.
- [Google Scholar]
- QT prolongation and torsades de pointes among methadone users: Reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf. 2005;14:747-53.
- [Google Scholar]
- Evaluation of QTc Interval Prolongation Among Patients With Cancer Using Enteral Methadone. Am J Hosp Palliat Care. 2019;36:177-84.
- [Google Scholar]
- The effect of oral methadone on the QTc interval in advanced cancer patients: A prospective pilot study. J Palliative Med. 2010;13:33-8.
- [Google Scholar]
- Practical guide for using methadone in pain and palliative care practice. Indian J Palliat Care. 2018;24:S21-S29.
- [Google Scholar]
- Methadone is now available in India: Is the long battle over? Indian J Palliat Care. 2018;24:S1-3.
- [Google Scholar]
- Methadone as a first-line opioid in cancer pain management: A systematic review. J Pain Symptom Manage. 2018;55:998-1003.
- [Google Scholar]
- The use of low-dose methadone as add-on to regular opioid therapy in cancer-related pain at end of life: A national Swedish survey in specialized palliative care. J Palliat Med. 2020;23:226-32.
- [Google Scholar]
- Efficacy of low-dose and/or adjuvant methadone in palliative medicine BMJ Supportive & Palliative Care Published Online First. 2019 04 04 doi: 101136/bmjspcare-2018-001695
- [Google Scholar]
- Methadone as first-line opioid treatment for cancer pain in a developing country palliative care unit. Support Care Cancer. 2016;24:3551-6.
- [Google Scholar]






