Translate this page into:
Effect of Celiac Plexus Neurolysis for Pain Relief in Patients with Upper Abdominal Malignancy: A Retrospective Observational Study and Review of Literature
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Abdominal pain from primary cancer or metastatic disease is a significant cause of pain for patients undergoing treatment for the disease. Patient's pain may be resistant or non-responsive to the pharmacological management, hence minimal invasive pain intervention like celiac plexus neurolysis or splanchnic nerve rhizolysis may be required to relieve pain of such patients.
Objective:
The aim of this retrospective study is to assess the effect of celiac plexus neurolysis for pain relief in patients with upper gastro-intestinal malignancies.
Study Design:
This is a retrospective, observational study with short review.
Methods:
This retrospective observational study was done in the Pain Medicine unit from 2016 and November 2018. Ninety-four patients with upper abdominal malignancy and unrelenting pain, non-responsive or poorly responsive to pharmacological treatment as per WHO ladder of analgesics, received fluoroscopy-guided celiac plexus neurolysis (CPN). All the patients underwent celiac plexus neurolysis through Trans-Aortic approach and the primary outcome measure was pain as assessed with Visual Analogue Scale (VAS) ranging from 0 to 10; the secondary outcome measures were morphine consumption per day (M), quality of life (QOL) as assessed by comparing the percent of positive responses and complications, if any. These were noted and analyzed prior to intervention and then on day 1, and months 1, 2, 3, 4, 5, 6 following CPN.
Results:
Follow up was completed 6 months after the procedure. VAS score, daily morphine consumption, and the quality of life showed improvement for the duration of the study. There was some relapse in pain and deterioration in QOL during the fourth to sixth month of pain intervention due to disease progression. Some transient known side effects also occurred.
Conclusion:
Trans-Aortic celiac plexus neurolysis with low volume of alcohol is a safe procedure providing up to 6 months of pain relief and is an effective, well established, minimally invasive procedure for abdominal pain due to primary malignancy or metastatic spread.
Keywords
Celiac plexus neurolysis
quality of life
transaortic approach
visual analog scale score
INTRODUCTION
Celiac plexus neurolysis (CPN) is the chemical neurolysis of the visceral afferent fibers that transmit pain from the upper abdominal viscera and is also recommended by the WHO cancer pain relief ladder.[1]
Pharmacological relief of pain from upper gastrointestinal cancer begins with nonopioid drugs such as paracetamol, “stepping up” to weaker opioids such as tramadol, and subsequently, more powerful opioids such as morphine or fentanyl. However, certain adverse effects of opioids such as nausea, constipation, somnolence, addiction, confusion, or respiratory depression mark the limitation of these drugs and thus somehow led to failure in achieving adequate analgesia. In these situation minimally invasive pain interventions like CPN may decrease the need of opioids and associated side effects.[23]
In this retrospective observational study, we have analyzed the efficacy of CPN in providing pain relief to patients suffering from upper abdominal malignancy.
History
Celiac plexus and splanchnic nerves neurolysis with local anesthetic were introduced as early as in 1914, primarily for surgical anesthesia. However, the introduction of neuromuscular blocking drugs into clinical practice of anesthesia led to the disfavor of celiac plexus block among anesthesiologists for surgical anesthesia.[4]
However, as the pain medicine specialty under anesthesiology emerged in the later quarter of the last century, CPN was finally introduced to palliate abdominal pain secondary to a variety of etiologies.
In 1914, Kappis introduced a percutaneous technique for splanchnic nerve blockade and CPN.
In 1946, Pitkin overviewed the status of celiac plexus blockade for surgical anesthesia and concluded that its utility was not beyond an experimental tool.
In 1947, Gage and Floyed described the use of CPN in alleviating the pain of pancreatitis.
In 1957, Bridenbaugh et al. used CPN to treat pain secondary to abdominal malignancies; in the same year, Jones introduced “alcohol induced neurolysis” of splanchnic nerves.
In 1965, Moore further modified the original Kappis's technique and established CPN as an important tool in pain management practice.
In 1971, Gorbitz (by the use of plain X-ray to facilitate CPN) related radiology to pain management practice.
In 1979, Hegedeus stressed the importance of fluoroscopic guidance in ascertaining correct needle placement and radio-contrast material spread. Moore/Hagga recommended a computed tomography scan to facilitate CPN.
Anatomy
The celiac “plexus” is the largest plexus of the sympathetic nervous system, innervating the upper abdominal organs (pancreas, gallbladder, diaphragm, liver, spleen, adrenal glands, kidneys, abdominal aorta, mesentery, stomach, small bowel, ascending colon, and the proximal portion of the transverse colon).
The celiac plexus is situated within the retroperitoneal space posterior to the stomach and pancreas, close to the celiac axis, and it is separated from the vertebral column by the crux of the diaphragm. It comprises a dense network of ganglia around the aorta, with considerable variability in size (0.5–4.5 cm), number (7–11), and position (from the T12–L1 disc space to the middle of the L2 vertebral body). The left celiac plexus is typically located more caudally than its counterpart on the right. Celiac neurolysis may target either the plexus or the ganglia.
The preganglionic sympathetic fibers of the celiac plexus are grouped into greater (T5–10), lesser (T10–11), and least (T12) splanchnic nerves, and the plexus also receives parasympathetic fibers from the celiac branch of the right vagus nerve.[5]
Celiac plexus neurolysis approaches
Multiple techniques of CPN have been described in the literature:[67]
-
Posterior (retrocrural approach)– classic
-
Transcrural approach
-
Transintervertebral disc approach
-
Transaortic approach
-
Anterior approach.
METHODS
This is a retrospective, observational study of patients treated and evaluated at the Pain clinic under the Department of Anesthesiology, Critical Care and Pain Medicine in Dr. Ram Manohar Lohia Institute of Medical Sciences, Lucknow, India, between November 2016 and November 2018. The patient list was generated using Operation theatre (OT) data.
Review of the patient records included the routine details on all patients in the pain medicine outpatient department prior to and following any pain procedure with a standard, detailed questioning and charting by the treating pain physician. The detailed data were thus tabulated on an Excel spreadsheet for the study. Procedures were done in a dedicated “Pain Medicine OT” under monitored anesthesia care.
Patients reviewed in the study were suffering from cancer originated pain from either primary upper abdominal malignancies or metastatic spread and resistant to oral analgesic and standard therapy, as per the WHO ladder. Detailed history and physical examination, an evaluation of each patient, were done before the pain intervention. The patient's subjective pain details such as location, radiation, and aggravating factors were recorded.
As per the local practice, patients with contraindications for regional blockade such as coagulopathy, infection at the entry point, and/or sepsis, ascites, and altered mental status did not undergo the procedure. For this study, medical records of the patients underwent transaortic unilateral CPN were scrutinized, and the data regarding Visual Analog Scale score (VAS score), quality of life (QOL), and oral morphine consumption were extracted and analyzed, at different time intervals as mentioned before.
Assessment of the VAS score,[8] QOL-C30,[9] and consumption of morphine[10] was done preintervention, postintervention, and 1-, 2-, 3-, 4-, 5-, and 6-month postintervention or end of patient's life, which is a routine data in our pain clinic.
Informed consent was obtained for each patient. Intravenous (IV) access for IV fluids was achieved before the intervention. Indian society of Anesthesiologist (ISA) standard vital monitoring including noninvasive blood pressure monitoring, Electrocardiography, pulse-oximetry etc. were employed in each patient. The “trans-aortic” approach for CPN was done in all patients with a patient in the prone position and a c-arm fluoroscope in position, L1 vertebrae was localized in scout anteroposterior (AP) view [Figure 1], then a “left sided” tilt of fluoroscope was done till the left-sided transverse process of L1 vertebrae merged with the L1 vertebral body [Figure 2]. Then, a 22 G, 15 cm spinal needle was inserted from the junction of the tip of L1 transverse process on the left side in a “gun-barrel view” and advanced until it penetrated the aortic wall suggested by feeling of loss of resistance and blood expulsion from the needle hub after removing needle stylet. Under continuous lateral fluoroscopic view; water soluble (omnipaque -300) dye was injected with advancement of needle till it crosses to anterior aortic wal [Figure 3]. After confirmation of satisfactory dye spread in AP view [Figure 4] and following negative aspiration, 5 ml of 1% lignocaine was administered, followed after 5 min by 15 ml of 100% alcohol.

- “Antero-posterior view”

- “Oblique view”

- Showing “wash-off” of dye in lateral view
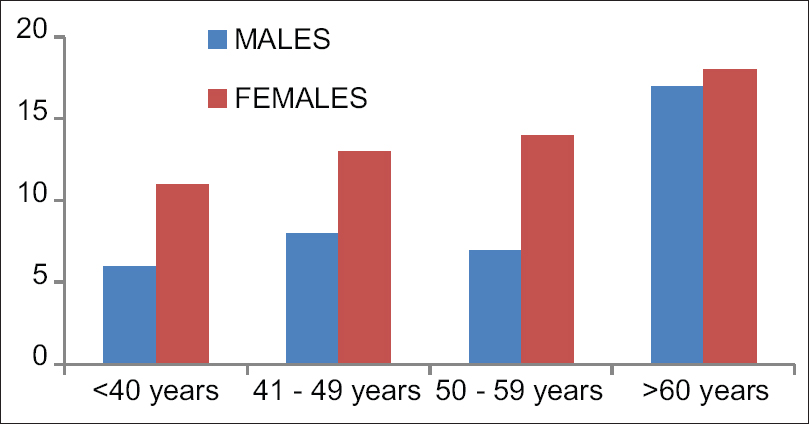
- Showing demographic histogram
The patients were then asked to remain prone for 30 min to avoid the unwanted neuroforaminal spread of injectate. Patients were monitored in a recovery room for 1 h for any adverse events and then were discharged once the hospital postanesthesia care discharge criteria were met. Patients were usually discharged home on the same day with a caregiver and called within 24 h.
Statistical analysis
Data collected were entered into Microsoft Excel® for subsequent statistical analysis. Association between nominal variables such as gender and age group was assessed using the Chi-square test. Age group-wise difference in duration of pain was statistically analyzed using one-way analysis of variance. Variables measuring VAS scores and QOL were presented as mean ± standard deviation (SD) and difference between means was statistically analyzed using a Paired t-test.
RESULTS
Ninety-four patients suffering from advanced upper abdominal malignancy underwent CPN for the alleviation of pain during November 2016–November 2018. Transaortic approach of CPN was used in all patients.
All the patients had failed conservative management including large doses of opioids, nonsteroidal anti-inflammatory drugs, adjuvant medicines, and physical therapy. All the patients were in various stages of cancer and were in progression states and had uncontrolled pain (including average pain score with SD). The cause of abdominal pain was primary abdominal cancers such as pancreatic, gastric, liver, gallbladder, and distant metastasis to the abdomen. The intraoperative period was uneventful with an expected intraoperative decrease in the mean arterial blood pressure >20% from baseline (10.4% cases, 10 out of 94 patients), responding to IV fluid therapy. All patients tolerated the procedure well, without any serious adverse events or complaints of an increase in pain score. The average procedure duration was 15.30 min (10.00–19.5 min). The postoperative period was uneventful with no significant procedure-related events.
Data from 94 patients regarding pain, QOL, and morphine equivalents consumption were collected and analyzed in this retrospective study. VAS scores, QOL-C30, and morphine equivalents consumptions were recorded at preoperative and postoperative, as well as after 1 month and then every month until the end of the 6 months or patient death. Due to higher mortality rates of advanced cancer origin diseases, complete follow-up was limited to only 27 patients who were alive until the completion of 6-month follow-up of the study, whereas the data of the rest of the patients (67 out of 94) were assessed until their demise.
There was no difference among the groups as regards to age, sex, and duration of pain [Table 1 and Figure 4]. Diagnosis of patients is presented as numbers [Table 2].
| Age groups | <40 | 41-49 | 50-59 | >60 |
|---|---|---|---|---|
| Males | 6 | 8 | 7 | 17 |
| Females | 11 | 13 | 14 | 18 |
| Duration of pain (months) | 2.4±0.4 | 2.8±0.8 | 3.6±1.0 | 4.0±1.2 |
| Diagnosis | Number of cases |
|---|---|
| Carcinoma gallbladder | 49 |
| Cholangiocarcinoma | 4 |
| Carcinoma stomach | 15 |
| Hepatic carcinoma | 7 |
| Carcinoma pancreas | 19 |
The mean VAS score preoperatively was 8.4 ± 1.6. The mean VAS scores were reduced after the procedure and during the follow-ups. Pain at postoperative and follow-up was significantly reduced compared to preoperative, P < 0.001 [Table 3]. However, 4th month onwards, VAS pain scores in the study patients were statistically significantly comparable to first 3 months' VAS score.
| Follow-up duration | Number of patients (n) | QOL score | P |
|---|---|---|---|
| Preprocedure | 94 | 32.02±5.01 | <0.001 |
| Day 1 | 33.21±5.37 | ||
| Postprocedure | 85 | 32.64±4.56 | <0.001 |
| Month 1 | 37.34±6.72 | ||
| Postprocedure | 70 | 33.14±4.50 | <0.001 |
| Month 2 | 40.13±6.48 | ||
| Postprocedure | 57 | 33.65±4.66 | <0.001 |
| Month 3 | 40.28±1.09 | ||
| Postprocedure | 39 | 34.82±4.49 | <0.001 |
| Month 4 | 41.28±7.29 | ||
| Postprocedure | 32 | 35.44±4.44 | <0.001 |
| Month 5 | 37.50±8.65 | ||
| Postprocedure | 28 | 35.64±4.62 | <0.001 |
| Month 6 | 31.64±10.08 |
QOL: Quality of life
The preoperative mean morphine consumption was 120 mg/24 h. The mean morphine consumption was reduced during the follow-up. The reduction rates of morphine consumption at follow-ups were significant compared to preoperative, P < 0.001 [Figure 5].

- Showing mean MORPHINE consumption comparing from preprocedural up to 6-month follow-up
The preoperative mean QOL was statistically comparable with day 1 post procedure and with 1, 2, 3, 4,5 and 6 months. Initially, there was improved overall QOL of almost all cases. However, many cases were in advanced stage, thereby assessment of QOL was affected due to the advancement of the disease [Table 4].
| Duration | Number of patients (n) | VAS score | P |
|---|---|---|---|
| Preprocedure | 94 | 8.4±1.6 | |
| Postprocedure Day 1 | 94 | 2.6±1.2 | <0.001 |
| Postprocedure Month 1 | 85 | 4.0±1.2 | <0.001 |
| Postprocedure Month 2 | 70 | 4.4±0.8 | <0.001 |
| Postprocedure Month 3 | 57 | 4.2±1.0 | <0.001 |
| Postprocedure Month 4 | 39 | 5.2±0.8 | <0.001 |
| Postprocedure Month 5 | 32 | 5.6±1.2 | <0.001 |
| Postprocedure Month 6 | 27 | 5.4±1.0 | <0.001 |
VAS: Visual Analog Scale
Complications and expected side effects included transient diarrhea in 12 patients lasting up to 2 weeks. Three patients had orthostatic hypotension with postural effects lasting 4 weeks and were required to see a cardiologist for a blood pressure medication regimen adjustment.
DISCUSSION
CPN is the most widely used interventional procedure for upper abdominal cancer pain relief with demonstrated efficacy for patients with malignant and chronic nonmalignant pain. It has been shown to provide a long-lasting benefit for 70%–90% of patients with pancreatic and intra-abdominal cancers with the benefit ranging from 50 days till up to the time of death.[1112]
As per Tewari et al.,[13] retrocrural CPN provides superior pain relief along with a reduction in morphine consumption as compared to transaortic CPN in patients with pain due to upper abdominal malignancy, whereas the positive effect of transaortic CPN was demonstrated by many studies,[11] Lillemoe et al.,[14] in 1993 in double-blind randomized study, compared chemical splanchnicectomy with 50% alcohol on patients with unresectable cancer pancreas with placebo, pain relief was significantly superior in the CPN-received patients compared with placebo. In a prospective, randomized study, Ischia et al.[15] evaluated the efficacy of three different approaches of neurolytic celiac plexus block in pancreatic cancer. Of 61 patients with pancreatic cancer pain, 29 (48%) experienced complete pain relief after the transaortic neurolysis. The remaining 32 patients (52%) required further therapy for residual visceral pain due to technical failure in 15 patients and neuropathic/somatic pains in 17 patients. The rate of initial pain relief immediately after intervention with the conventional method was 94%.
Positioning the needle tip adjacent to the anterolateral wall of the aorta in this technique may have facilitated the maximum spread of alcohol which was well explained by R. P. Libermann et al.[16] through a modified transaortic approach.
Rahman et al.[17] came with a retrospective study in which they concluded that low volume of alcohol has proven benefit in QOL, VAS score as well as less chances of complication as compared to the high volume of alcohol being used in bilateral retrocrural CPN; in our study also, we have used smaller volume of neurolytic agent due to unilateral transaortic approach. Furthermore, Eisenberg et al.[18] suggested that CPN has long-lasting benefits for 70%–90% of patients with pancreatic and other intra-abdominal cancers, regardless of the technique used. Bridenbaugh et al.[19] concluded that a simple procedure like transaortic CPN proved effective in controlling the pain without any serious complications, and the results of our retrospective trial are also similar to this study. Amr and Makharita[20] found that the analgesia induced by the celiac neurolysis after medically controlling pain was better and more sustained when compared with the outcome on performing a celiac neurolysis at a high VAS score >7.
Post CPN, any residual or break-through pain was managed as per the WHO analgesic ladder. The differences observed in morphine consumption indicate that post procedure, satisfactory pain relief was achieved with the use of simple analgesics and weak opioids such as tramadol, obviating the need to consume high doses of strong opioids such as morphine. The maximum benefit achieved up to the 3rd month post procedure. Frequent side effects such as dry mouth, drowsiness, constipation, and nausea and vomiting have often been reported with the use of morphine in cancer patients, and reduction in morphine requirement is known to improve social and cognitive scales and hence facilitate the better end of life care. The reduction of morphine consumption was thus a valuable advantage of the transaortic CPN, as evident by our results.
The importance of QOL issues for cancer patients is well recognized, and over the past several decades, numerous studies have addressed the physical, emotional, social, and sexual well-being of cancer patients. A number of cancer-specific QOL measures have been developed and various previous studies evaluating CPN in pancreatic malignancies have demonstrated improved physical, emotional, and social well-being in patients receiving CPN. The results of our study also support improved QOL after CPN, at least in initial months. However, progression of the disease plays a major counterpart role.
CPN is usually a safe procedure with rare serious complications.[21] These complications are usually caused due to either chemical or traumatic injury to the surrounding structures. The most common complication that we observed was a transient backache at the site of injection. Previously, hypotension secondary to sympathetic denervation has been documented in almost one-third of patients and self-limiting diarrhea in about 40% of patients as a result of unopposed parasympathetic activity. These were also observed in our studied patients as well. Various other complications have also been reported such as shoulder pain, dysesthesia, impaired ejaculation, diaphragmatic paralysis, and pneumothorax. These complications are uncommon and were not encountered in any of our patients.
CONCLUSION
Transaortic CPN is a simple and effective technique for upper abdominal malignancy pain. The results of CPN can be better if employed early in the course of disease.
Limitation
Ours is a retrospective study of only one technique employed. A randomized head-to-head trial of different techniques will provide more insight into the optimum and best technique of CPN.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- The modified WHO analgesic ladder: Is it appropriate for chronic non-cancer pain.? J Pain Res. 2020;13:411-7.
- [Google Scholar]
- Clinical pharmacology: principles of analgesic drug management. In: Sykes N, Bennett MI, Yuan CS, eds. Clinical Pain Management: Cancer Pain Vol 2. (2nd ed). London: Hodder Education; 2008. p. :103-122.
- [Google Scholar]
- Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer-a randomized controlled trial. JAMA. 2004;291:1092-9.
- [Google Scholar]
- Celiac plexus block: An anatomical study and simulation using computed tomography. Radiol Bras. 2014;47:283-7.
- [Google Scholar]
- Sympathetic blockade. In: Smith HS, ed. Current Therapy in Pain. Elsevier Saunders; 2009.
- [Google Scholar]
- Neurolytic celiac plexus block for pancreatic cancer pain: A review of literature. Indian J Pain. 2013;27:121-31.
- [Google Scholar]
- Quality of life in cancer patients receiving palliative care. Indian J Palliat Care. 2010;16:36-43.
- [Google Scholar]
- The use of morphine to treat cancer-related pain: A synthesis of quantitative and qualitative research. J Pain Symptom Manage. 2010;39:139-54.
- [Google Scholar]
- Comparative evaluation of different volumes of 70% alcohol in celiac plexus block for upper abdominal malignsancies. South Asian J Cancer. 2016;5:204-9.
- [Google Scholar]
- Comparative Evaluation of Retrocrural versus TransaorticNeurolyticCeliac Plexus rhizolysis for pain relief in patients with upper gastro-intestinal malignancies. Reg Indian J Palliative Care. 2016;22:301-6.
- [Google Scholar]
- Chemical splanchnicectomy in patients with unresectable pancreatic cancer.A prospective randomized trial. Ann Surg. 1993;217:447-55.
- [Google Scholar]
- Three posterior percutaneous celiac plexus block techniques.A prospective, randomized study in 61 patients with pancreatic cancer pain. Anesthesiology. 1992;76:534-40.
- [Google Scholar]
- Celiac plexus neurolysis with the modified transaortic approach. Reg RSNA. 1990;175:274-6.
- [Google Scholar]
- Low volume neurolytic retrocruralcoaliac plexus block. Pain Physician. 2018;21:497-504.
- [Google Scholar]
- Neurolytic celiac plexus block for treatment of cancer pain: A meta-analysis. Anesth Analg. 1995;80:290-5.
- [Google Scholar]
- Management of upper abdominal cancer pain: treatment with celiac plexus block with alcohol. JAMA. 1964;190:877-80.
- [Google Scholar]
- Comparative study between 2 protocols for management of severe pain in patients with unresectable pancreatic cancer: One-year follow-up. Clin J Pain. 2013;29:807-13.
- [Google Scholar]
- Complications of joint, tendon, and muscle injections. Tech Reg Anesth Pain Manag. 2007;11:141-7.
- [Google Scholar]






