Translate this page into:
Indian Society for Study of Pain, Cancer Pain Special Interest Group Guidelines, for the Diagnosis and Assessment of Cancer Pain
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The Indian Society for Study of Pain (ISSP), Cancer Pain Special Interest Group (SIG) guidelines, for the diagnosis and assessment of cancer pain in adults provide a structured, step-wise approach which will help to improve the management of cancer pain and to provide the patients with a minimally acceptable quality of life. The guidelines have been developed based on the available literature and evidence, to suit the needs of patient population and situations in India. A questionnaire based on the key elements of each sub draft addressing certain inconclusive areas where evidence was lacking, was made available on the ISSP website and circulated by E-mail to all the ISSP and Indian Association of Palliative Care (IAPC) members. We recommend that a comprehensive pain assessment of all the patients should be conducted before initiating treatment. The patients should be educated about all the available pain control interventions. For assessing cancer pain, unidimensional tools such as Numeric Rating Scale, Visual Analog Scale, and Visual Rating Scale should always be used routinely. Patients with cancer pain should routinely be screened for distress and other psychological disorders, using the Patient Health Questionnaire-9. The most reliable assessment of pain is patients' self-reporting.
Keywords
Assessment of cancer pain
Cancer Pain Management Guidelines
Cancer Pain Special Interest Group
diagnosis
Indian Association of Palliative Care
Indian Society for Study of Pain
INTRODUCTION
Worldwide, low-and middle-income countries are experiencing significant increases in the rates of noncommunicable diseases, including cancer.[1] In India, more than one million new cases of cancer are diagnosed each year, and it is estimated that the cancer burden in India will almost double during the coming 20 years.[2] The incidence of pain in advanced stages of cancer approaches 70%–80%.[3] A meta-analysis of epidemiological studies on cancer pain revealed that the pain prevalence rates were 39.3% (95% confidence interval [CI]: 33.3–45.3) after curative treatment; 55.0% (95% CI: 45.9–64.2) during anticancer treatment; 66.4% (95% CI: 58.1–74.7) in advanced, metastatic, or terminal disease; and 50.7% (95% CI: 37.2–64.1) in all cancer stages.[4] It was also shown that over 38.0% of all cancer patients experienced moderate-to-severe pain (pain score >4/10).[4] In a study done at four regional cancer centers in India, nearly 88% of the patients reported experiencing pain for about 7 days, and approximately 60% reported that their worst pain was severe.[5]
With advancements in oncologic care, there is an increase in the number of cancer survivors, which also increases the number of patients suffering from pain due to treatment or disease, or a combination of both.[6] Approximately 5%–10% of those in survivorship present with chronic severe pain that interferes significantly with their functioning.[7]
Although pain is often the primary presenting symptom of cancer and despite the presence of guidelines and the availability of opioids, cancer pain still remains undertreated. In a systematic review[8] published in 2014 using the Pain Management Index, approximately 1/3rd of the patients did not receive appropriate analgesia proportional to their pain intensity (PI), as advised by the World Health Organization (WHO) analgesic ladder.
The WHO states that “Drug treatment is the mainstay of cancer pain management.”[9] Pain treatment using WHO guidelines provides pain relief in majority of patients, though an effective pain relief may take a long time in a third of the patients. Some advocate a fourth step of interventional therapies to the ladder and recommend using a flex approach rather than a step-wise approach for optimal pain relief.[10] Cancer pain management guidelines have been published by the clinical practice guidelines management of cancer pain (Malaysian Cancer Pain Guidelines, July 2010), Ministry of Health Clinical Practice Guidelines 5/2003 (Singapore), Scottish Intercollegiate Guidelines Network 2008, European Society of Medical Oncology cancer pain guidelines (2018), National Comprehensive Cancer Network (NCCN) cancer pain guidelines – Adult Cancer Pain 2018 (NCCN, 2018), American Society of Clinical Oncology guidelines, and British Pain Society Guidelines.[11121314151617] Although these are truly exemplary guidelines, they take into account the scope of practice only in the respective countries. As the patient population is different with respect to the Indian context, they may not work well. Conditions of medical practice are not only different in our country but are also variable depending on the type of institution/center that one works in. The WHO and the International Association for the Study of Pain have stated that “Pain Relief is a Basic Human Right.”[18]
These guidelines are developed to improve the management of cancer pain and to provide the patients with a minimal acceptable quality of life.
METHODS
The Executive Committee of the Indian Society for Study of Pain (ISSP) appointed two coordinators for the Cancer Pain Special Interest Group (SIG). The coordinators in turn appointed three-tier systems for the development of these guidelines. The first tier comprised the Guidelines-Development Committee (GDC) [Appendix I], the second tier comprised the Internal Review Committee [Appendix II], and the third tier comprised the External Review Committee [Appendix III]. The Cancer Pain SIG formed a multidisciplinary working group comprising the members of and experts appointed by the cancer pain SIG coordinators. They consisted of pain medicine specialists, palliative medicine physicians, oncologists, pain nurses, and psychologists.
Literature search [Appendix IV] was carried out using PUBMED, MEDLINE, COCHRANE DATABASE, GOOGLE SCHOLAR, and OVID Search engine. The search included studies published in English language until November 2018. Additional articles were retrieved by cross-referencing and hand searching. The guidelines-developing committee consisted of eight members including the coordinators of the Cancer pain SIG of ISSP. The committee was further divided into five subcommittees to draft each of the six guidelines (diagnosis and assessment, pharmacological management, interventional management, complementary management, and palliative aspects of cancer pain). The members were in communication through E-mails. Each article was reviewed by at least two members of the GDC. In addition, cancer pain management guidelines of various societies[11121314151617] were reviewed. The five drafts of these guidelines were presented during the 34th Annual Conference of Indian Society for Study of Pain (ISSPCON 2019) at Bengaluru, where members of ISSP from all over India participated and gave their consensus-based opinion. A questionnaire [Appendix V] based on the key elements of each sub draft addressing certain inconclusive areas where evidence was lacking, was circulated during the meeting and also was made available on the ISSP website and circulated by E-mail to all the ISSP and Indian Association of Palliative Care (IAPC) members. The responses of the respondents to the questionnaire were analysed. Where evidence is lacking, recommendations were made by consensus, following extensive discussion among the committee members and considering the results of the questionnaire.
What is cancer pain?
Pain attributable to cancer or its therapy in a patient with cancer is considered as cancer pain. It includes all components of the experience of pain (physical, behavioural, cognitive, emotional, spiritual, and interpersonal aspects).[19] These multidimensional aspects of pain should be followed while assessing and managing patients with cancer pain.
A comprehensive assessment should therefore include:
-
Physical manifestations of pain
-
Interference with activities of daily living
-
Psychosocial factors (anxiety, mood, cultural influences
-
Fears, effects on interpersonal relationships, etc.)
-
Spiritual aspects.
Why should we assess pain?
Accurate assessment and diagnosis of the type of pain, its severity, and effect on the patient are necessary to plan treatment or interventions.[19] Etiology of pain should be identified and should not or cannot always be attributed to the cancer itself. Approximately, 5%–10% of patients with cancer may report pain due to other conditions.[20] Careful history, active listening, and a detailed physical examination will help diagnose the type of pain and understand the patient's expectations, which, in turn, helps to plan pain therapy and provide pain relief to a comfortable level in order to perform certain activities with minimal and bearable pain. Assessment of pain helps in the selection of drug and route for the treatment of pain. It also helps to evaluate the effectiveness of the treatment given for pain relief.
Who should assess patient's pain?
The patient himself/herself is the most reliable assessor of the pain and should, whenever possible, be the prime assessor of his or her pain as he/she can better relate to its impact on his/her quality of life.[21]
Due to frailty, cognitive impairment, or communication deficits, many patients are not able to express their problems and find completion or filling of pain scoring tools difficult. In such cases, family or health-care professionals may help in extracting or eliciting the patient's problems.
How should pain be assessed?
Careful history taking and engaging in listening nonjudgmentally and attentively help to diagnose the type of pain and this in turn dictates the therapy.
For assessing cancer pain, unidimensional tools such as Numeric Rating Scale (NRS), Visual Analog Scale (VAS), and Verbal Rating Scale (VRS) should always be used routinely. Patients with cancer pain should routinely be screened for distress and other psychological disorders, using the Patient Health Questionnaire-9 (PHQ-9).
The PI will determine which step of the therapeutic intervention of the WHO ladder would need to be used.
How often should the pain be assessed?
The key to successful cancer pain control is a regular review to identify the effectiveness of the treatment that is being implemented. Hence, patients should be reviewed regularly. The severity of pain and the associated distress determines the frequency of the assessment, which is conducted daily or more often when pain is not well controlled.
History
Detailed history of pain:
-
Site/location of pain: A patient may have more than one site of pain
-
Types of pain: A patient may have more than one type of pain. Each should be assessed including those not due to cancer
-
Intensity of pain: Based on patient's self-report using:
-
Timing of pain:
-
Onset
-
Duration
-
How has pain changed over time
-
Intermittent or persistent
-
Spontaneous or with some activity
-
Whether controlled by medication and recurs at certain times or at the end of dose interval.
-
-
Characteristics of the pain (pathophysiology): Allow patient to describe the pain using pain descriptors as well as in his/her own words
-
Nociceptive pain: Injury to somatic and visceral structures with activation of nociceptors
-
Somatic: Sharp, well localized, throbbing, aching, stabbing, pressure like
-
Visceral: Diffuse, aching, cramping, gnawing
-
Neuropathic pain: Injury or diseases affecting the peripheral or central somatosensory nervous system. Described as burning, pricking, pins and needles, numb, sharp, shooting, and tingling.
-
-
Aggravating and relieving factors; All factors which make the pain worse or relieve it (e. g., cough, exercises, and walking)
-
Interference with daily activities: General activity, sleep, mood, appetite using a validated assessment tool (e. g., The Brief Pain Inventory Short Form [BPI])
-
Etiology of pain: Is the pain caused by:
-
Cancer
-
Cancer therapy: Radiation, chemotherapy, surgery, or procedure-related pain
-
Unrelated to cancer.
-
-
Analgesic drug history: Current/past pain medication
-
What medications?
-
What dose, how often, and how long?
-
Who prescribed?
-
Why was it prescribed?
-
What was the response?
-
Pain relief and amount of pain relief
-
Side effects and treatment of side effects
-
Reason for discontinuation.
-
-
Patient's and family's knowledge, beliefs about pain, and its treatment should be assessed and discussed which include:
-
Concerns related to fear of addiction
-
Tolerance
-
Side effects
-
Fear that use of opioid means the end stage of the disease
-
Nearly 28%–38% of all cancer pain patients do not comply with their analgesic regimes[22] and hence these issues which hinder adherence should be addressed.
Educational interventions can change patients' cancer pain-related beliefs and behaviour, as shown by two randomized controlled trials carried out in Taiwan.[2223]
-
-
Patients' goals and expectations regarding pain management
-
Oncologic treatment includes:
-
Chemotherapy: Ongoing and prior
-
Radiation therapy
-
Surgery.
-
-
Medical history
-
Presence of other illnesses/comorbidities such as diabetes mellitus and kidney disease
-
Preexisting chronic pain.
-
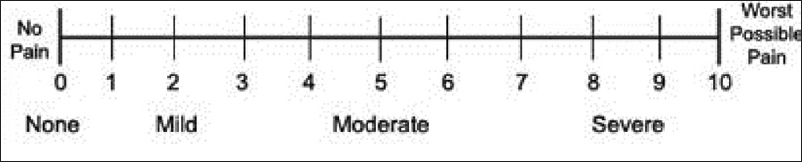
- A 0–10 Numerical Rating Scale

- Wong–Baker Faces Pain Rating Scale
Physical examination
In addition to routine physical examination, it is essential to perform a comprehensive neurological and musculoskeletal examination as well.
-
General physical examination
-
General constitution
-
Height, weight, and body mass index
-
Vital signs: Pulse, blood pressure, respiratory rate, temperature and pain score (VAS)
-
Appearance, development, deformities, nutrition, grooming, etc., to be noted.
-
-
Skin and mucous membrane
-
Check for colour, temperature, rash, soft-tissue edema, and pressure sores in bedbound patients
-
Trophic changes of skin, nails, and hair are seen in complex regional pain syndromes, especially in advanced stages
-
Oral mucous membranes for candidiasis/mucositis: Special attention to be given for the evaluation of lymphedema in postsurgical/advance metastatic/haemato-oncological patients, especially lymphomas
-
Check for any possible site of infection, especially in patients with diabetes, vascular disease, and peripheral neuropathy. The infection must be treated and risk should be assessed before implanting devices such as spinal cord stimulators or infusion pumps.
-
-
Pain behaviour
-
Facial expression, colour, and grimacing should be noted
-
Some patients may in their attempt to convince the physician and may grunt, moan, twitch, grab the painful area, and exaggerate their posture to show that they are suffering from a great deal of pain.
-
-
Cardiovascular system
-
Check for murmurs and irregular rhythm.
A systolic murmur could point to an aortic stenosis, such a patient may not tolerate hypovolemia and rapid vasodilation as they may occur with neuraxial local anaesthetics and sympathetic or celiac plexus block.
An irregular rhythm may mean atrial fibrillation and such patients may be on anticoagulants. History of cardiac comorbidities and stroke and use of anticoagulants and antiplatelet drugs should be confirmed.
-
-
Check capillary refill or return
-
Respiratory system
-
Abnormal breath sounds such as crackles may be a sign of congestive cardiac failure
-
Rhonchi or wheezes are signs of chronic obstructive pulmonary disease
-
Patients should be optimized prior to any interventional procedure. Extreme caution should be exercised while conducting blocks around the chest cavity, as there is an increased risk of pneumothorax.
-
-
-
Neurological examination:
-
Mental status
-
Alertness level
-
Orientation to time, place, and person
-
General appearance
-
Behaviour and mood including suicidal ideations
-
Intellect including comprehension, attention, insight, and memory.
-
-
Motor system
-
Appearance of muscles: Check muscle bulk, tone, and spasm. Look for atrophy. Measure the circumference of both limbs at the calf and thigh levels. A ≥2 cm difference at the same level is indicative of atrophy
-
Tone
-
Strength: Test muscle strength in both upper and lower extremities
-
Gait: Gives information on muscle strength and impaired vestibular, cerebellar, or dorsal column function. It also informs if braces or assistive devices are needed for stability/safety (e. g. limit foot drop)
-
Look for latent weakness by asking patients to walk on their toes and heels. Heel walking is most sensitive for detecting the weakness of foot dorsiflexion. Toe walking helps detect early weakness of foot plantar flexion.
-
-
Sensory perception: Elicited by the application of various stimuli such as light touch, pin prick, temperature (hot and cold stimuli), and pressure/vibration to the suspect area. An ice cube or a freshly opened alcohol wipe may be used to elicit deficit in cold perception or to elicit cold allodynia.
The following may be observed in neuropathic pain conditions:
-
Hyperesthesia: Increased sensitivity to stimulation, excluding special senses
-
Dysesthesia: An unpleasant abnormal sensation, either spontaneous or evoked
-
Allodynia: Pain caused by a stimulus that is normally not painful
-
Hyperalgesia: A heightened response to a stimulus that is normally painful
-
Hyperpathia: Clinical symptom of certain neurological disorders wherein nociceptive stimuli evoke exaggerated levels of pain. Therefore, the threshold for pain is decreased
-
Summation: A repetitive pinprick stimulus at intervals of < 3 s, resulting in a gradually increasing sensation of pain with each subsequent stimulus
-
Deep tendon reflexes: Tendon reflexes that are not under voluntary control and alterations indicate signs of neurological dysfunction. Pathological reflexes such as Babinski (foot) and Hoffmann (hand) must be tested. Positive tests indicate upper motor neuron dysfunction
-
Cranial nerve function: The 12 cranial nerves relay messages between the brain and the head and neck. They mediate motor and sensory functions including vision, hearing, smell, tongue, and vocal cord movements. The fifth cranial nerve or the trigeminal nerve is involved in trigeminal neuralgia, which requires assessment of facial sensations, jaw strength and movement, and corneal reflexes.
-
-
Musculoskeletal examination is also important:
-
Pain may be secondary to postural changes
-
Deconditioning may lead to muscle atrophy
-
The patient may adopt abnormal patterns of movement and have loss of range of movements in the spine and other joints
-
Bedbound patients need to be evaluated for deep-vein thrombosis.
-
All of which can result in loss of function and increasing pain and worsen trophic changes.
-
Specific examination of major joints
-
Note active and passive ranges of movements at all major joints. Any pain on palpation
-
Shoulders: Examine for brachial plexopathy with radicular pain down the arm into the hand. Also, check for inflammatory or adhesive capsulitis
-
Elbows: Signs of ligamentous or tendon inflammation
-
Wrists: For signs of carpal tunnel syndrome
-
Hands and fingers: For signs of arthritis
-
Knees: Tenderness and abnormal patellar tracking
-
Hips: For trochanteric bursitis
-
Ankles and feet: For plantar fasciitis.
-
-
Spine examination
-
Signs of kyphosis, scoliosis, and pelvic tilt
-
Palpate midline for tenderness over the spinous processes (collapse of vertebral bodies) and discs, paravertebrally for facet joint tenderness
-
Flexion, extension, lateral flexion, and rotation. Pain on flexion may suggest muscle spasm. Pain on extension/rotation or ipsilateral pain on lateral flexion may point to facet-related pain
-
Sacroiliac joint palpation: May be positive in case of inflammation. Straight leg raising or sitting leg extension resulting in pain radiating to the foot or in the contralateral low back may indicate lumbosacral nerve root irritation or compression from lumbar disc protrusion or spinal stenosis.
-
Diagnostic and investigation tools
These tools are only conducted if they help us in planning further management of pain. If patients are going to be treated empirically anyway (e.g. end of life), don't expose them to cost/pain of additional testing.
-
Complete blood count: White blood cells and platelet count (to know overall general condition of patient)
-
Coagulation profile: International normalized ratio (will help if planning for any interventions)
-
Renal function test: Serum creatinine (to consider nonsteroidal anti-inflammatory drugs)
-
Liver function test: Enzymes
-
Imaging
-
X-rays or radiographs provide details of bone structure and identify fractures
-
Bone scans are more sensitive in detecting skeletal pathology than X-rays. They help rule out:
-
Occult fractures, i.e. small fractures not visible on routine X-rays
-
Inflammatory processes including infection and tumours
-
A new vertebral compression fracture from an old one. The new fracture will show an uptake, whereas an old one will not.
A bone scan, however, has a low specificity and cannot differentiate between a tumour, an infection, and a fracture. Computed tomography (CT) or magnetic resonance imaging (MRI) helps characterize such lesions.
-
CT: Delineates the bony structures well
-
Positron emission tomography-CT will identify whether there is ongoing tumour activity
-
MRI: Uses no ionizing radiation. Provides superior contrast of soft tissues, especially neural tissues in comparison with CT.
The imaging needs to be read in conjunction of background history and examination findings.
All imaging findings do not require treatment and may not correlate with symptoms as well.
Pain assessment tools
Several pain assessment tools are used worldwide with a considerable amount of inconsistency between them while assessing cancer pain. There is no universally accepted tool for the assessment of cancer pain.
To overcome this problem, the European Association of Palliative Care has recommended the use of standardized pain assessment tools in palliative care in research and clinical practice.[24] These include the VAS, the Numerical Rating Scale (NRS), and the Verbal Rating Scale (VRS)
-
VAS – A 10-cm line [Figure 3] and end point descriptors. Patient marks the point on the line which best represents his/her PI
-
NRS – An 11-point scale [Figure 1], with 0 at one end represents “no pain” and 10 represents “worst possible pain”
-
VRS – Employs descriptors of PI [Figure 4] such as “none,” “mild,” “moderate,” and “severe.”

- The Visual Analog Scale

- The Verbal Rating Scale
The above-mentioned are unidimensional pain scales which evaluate only one dimension of pain that is the pain severity or intensity.
Multidimensional pain scales assess not only the intensity of pain but also give an insight into the interference of pain with various aspects of daily living such as general activity, sleep, mood, and appetite, to name a few.
The McGill Pain Questionnaire and the BPI are two commonly used multidimensional pain scales that incorporate the NRS and VRS which have been validated in different languages and cultures including India.[2526]
VAS, Numerical Rating Scale (NRS), and Verbal Rating Scale (VRS) are the most commonly used unidimensional assessment tools which are valid and adequately reliable.[27282930] There is a nonlinear relationship between cancer pain severity and functional interference,[31] with mild pain = 1–4, moderate pain = 5–6, and severe pain = 7–10 marked on NRS (BPI).
Psychological assessment
-
Presence of clinically significant psychological disorder, anxiety or depression, coping skills
-
Previous or current substance abuse
-
Risk of opioid misuse
-
Spiritual and religious beliefs that may affect pain and its management.
In a single-cohort study involving 194 Taiwanese cancer patients, it was shown that patients who have a stronger belief in the role of medication to control pain and weaker beliefs in their own ability to manage pain are more likely to adhere to medications. This study suggests that to enhance adherence to medications, pain beliefs should be assessed and integrated into pain management and patient education.[32] The PHQ-9 is a screening instrument with nine items, developed to measure depression. For each item, the patients are asked to assess how much they were bothered by the symptoms over the last 2 weeks. There are four answer options: not at all (0), several days (1), more than half of the days (2), and nearly every day (3). The sum score (range: 0–27) indicates the degree of depression, with scores of ≥ 5, ≥10, and ≥ 15 representing mild, moderate, and severe levels of depression, respectively. The PHQ-9 is comprised of items that measure several aspects of depression,[33] but that it is nevertheless useful to maintain the PHQ-9 as a unidimensional scale in practical applications.
Oncological emergencies
Consider whether pain is related to an oncologic emergency such as:
-
Bone fracture or impending fracture of weight-bearing bone
-
Neuraxial metastasis with nerve damage or threatened neural injury
-
Brain metastasis
-
Epidural metastasis (is impending spinal cord compression)
-
Leptomeningeal metastasis
-
Infection
-
Obstructed or perforated viscus.
CONCLUSION
The ISSP cancer pain SIG guidelines for diagnosis and assessment of cancer pain in adults emphasize the importance of comprehensive pain assessment along with patient education about the availability of various pain control interventions in the form of pharmacological and nonpharmacological methods. It also emphasizes on using unidimensional as well as multidimensional pain scoring tools along with screening of patients for distress and psychological disorders. The most important aspect of these guidelines is recommendation of patient participation and self-reporting of pain [Table 1].
| Recommendations | Level of evidence |
|---|---|
| A comprehensive pain assessment of all the patients should be conducted before initiating treatment (Grade C) | III |
| Patients should be educated about pain control interventions available to them (Grade C) | III |
| For assessing cancer pain, unidimensional tools like NRS, VAS, VRS should always be used routinely (Grade B) | II |
| Patients with cancer pain should routinely be screened for distress and other psychological disorders (PHQ-9) (Grade B) | II/III |
| The most reliable assessment of pain is patients self-reporting (Grade C) | III |
NRS: Numeric Rating Scale, VAS: Visual Analog Scale, PHQ-9: Patient Health Questionnaire-9, VRS: Verbal Rating Scale
We believe that the ISSP cancer pain SIG guidelines for diagnosis and assessment of cancer pain in adults will help pain specialists, anaesthesiologists, palliative care specialists, and others who are involved in cancer pain care, in the safe management of cancer pain and to provide the patients with a minimally acceptable quality of life.
Disclaimer
The contents of this publication are guidelines to clinical practice, based on the best available evidence at the time of development. These guidelines should neither be construed or serve as a standard of care.
These guidelines do not represent the minimum standard of practice, nor are they a substitution for good clinical judgment. These guidelines need to be used in conjunction with patient assessment and may be individualized as per patient need.
These guidelines were developed in 2018-2019 and may be reviewed again in 2024 or sooner, based on the availability of new evidences.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The ISSP cancer pain SIG guidelines' GDC would like to thank the President, Secretary, and the Governing Council of ISSP as well the Chairman of SIG. The ISSP cancer pain SIG guidelines' GDC would like to thank the members of the ISSP, the IAPC, and other anaesthesiologists who responded to the questionnaire and gave their valuable feedback which helped in the formulation of these guidelines.
The ISSP cancer pain SIG would like to wholeheartedly thank the Internal Review Committee [Appendix II] and the External Review Committee [Appendix III].
REFERENCES
- Non-communicable diseases in low- and middle-income countries: Context, determinants and health policy. Trop Med Int Health. 2008;13:1225-34.
- [Google Scholar]
- The growing burden of cancer in India: Epidemiology and social context. Lancet Oncol. 2014;15:e205-12.
- [Google Scholar]
- Cancer pain management in developing countries. Indian J Palliat Care. 2016;22:373-7.
- [Google Scholar]
- Update on prevalence of pain in patients with cancer: Systematic review and meta-analysis. J Pain Symptom Manage. 2016;51:1070-90e9.
- [Google Scholar]
- Predictors and prevalence of pain and its management in four regional cancer hospitals in India. J Glob Oncol. 2018;4:1-9.
- [Google Scholar]
- Quality of cancer pain management: An update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32:4149-54.
- [Google Scholar]
- Cancer Pain Relief With a Guide to Opioid Availability (2nd ed). Geneva: World Health Organization; 1996.
- Is the WHO analgesic ladder still valid.Twenty-four years of experience? Can Fam Physician. 2010;56:514.
- [Google Scholar]
- Clinical Practice Guidelines Management of Cancer Pain (Malaysian cancer pain Guidelines) MOH/P/PAK/20510(GU) Ministry of Health 2010
- 2003. Clinical Practice Guidelines 5/2003. Singapore: Ministry of Health; Available from: http://wwwgovsg/moh/pub/cpg/cpghtm
- Control of pain in adults with cancer: Summary of SIGN guidelines. BMJ. 2008;337:a2154.
- [Google Scholar]
- Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29:iv166-91.
- [Google Scholar]
- NCCN Clinical Practice Guidelines in Oncology: Adult Cancer Pain Ver 1:2018 National Comprehensive Cancer Network, Inc 2018
- Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:3325-45.
- [Google Scholar]
- Pain Relief as a Human Right Pain Clinical Updates Vol 12 International Association for the Study of Pain 2004:1-4.
- Assessment of cancer pain: A prospective evaluation in 2266 cancer patients referred to a pain service. Pain. 1996;64:107-14.
- [Google Scholar]
- Symptom Management in Advanced Cancer (2nd ed). London: Pitman; 1997.
- Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330:592-6.
- [Google Scholar]
- Overcoming patient-related barriers to cancer pain management for home care patients. A pilot study. Cancer Nurs. 2002;25:470-6.
- [Google Scholar]
- Long-term effectiveness of a patient and family pain education program on overcoming barriers to management of cancer pain. Pain. 2006;122:271-81.
- [Google Scholar]
- Pain measurement tools and methods in clinical research in palliative care: Recommendations of an Expert Working Group of the European Association of Palliative Care. J Pain Symptom Manage. 2002;23:239-55.
- [Google Scholar]
- Greek McGill Pain Questionnaire: Validation and utility in cancer patients. J Pain Symptom Manage. 2002;24:379-87.
- [Google Scholar]
- A validation study of an Italian version of the Brief Pain Inventory (Breve Questionario per la Valutazione del Dolore) Pain. 1996;65:87-92.
- [Google Scholar]
- Clinical evaluation of mild analgesics: The measurement of clinical pain. Br J Clin Pharmacol. 1980;10(Suppl 2):319S-27S.
- [Google Scholar]
- Reassessment of verbal and visual analog ratings in analgesic studies. Clin Pharmacol Ther. 1985;38:16-23.
- [Google Scholar]
- Cancer pain assessment in clinical trials. A review of the literature (1999-2002) J Pain Symptom Manage. 2005;29:507-19.
- [Google Scholar]
- The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4:2-1.
- [Google Scholar]
- What should be the optimal cut points for mild, moderate, and severe pain? J Palliat Med. 2007;10:1338-46.
- [Google Scholar]
- Relationship between pain-specific beliefs and adherence to analgesic regimens in Taiwanese cancer patients: A preliminary study. J Pain Symptom Manage. 2002;24:415-23.
- [Google Scholar]
- Assessment of depression severity with the PHQ-9 in cancer patients and in the general population. BMC Psychiatry. 2016;16:22.
- [Google Scholar]
APPENDIX I: GUIDELINES DEVELOPMENT COMMITTEE
Dr. Raghu S Thota, Dr. Dipasri Bhattacharya, Dr. Parmanand Jain, Dr. Sushma Bhatnagar, Dr. Aparna Chatterjee, Dr. Naveen Salins, Dr. Raghavendra Ramanjulu, Dr. Arif Ahmed
APPENDIX II: INTERNAL REVIEW COMMITTEE
Dr. Sukdev Nayak, Professor, Anaesthesia, AIIMS, Bhubaneswar Dr. Geeta Joshi, Chief Executive Officer, Community Oncology Centre, Ahmedabad Dr. Rakesh Garg, Associate Professor, Dr. BRAIRCH, AIIMS, New Delhi Dr. Umesh Mahantshetty, Professor, Radiation Oncology, Tata Memorial Centre (HBNI), Mumbai Dr. Kumar Prabhash, Professor, Medical Oncology, Tata Memorial Centre (HBNI), Mumbai Dr. Syed Nusrath, Consultant Surgical Oncologist, Basavatarakam Indo American Cancer Hospital and Research Institute, Hyderabad. Dr. Rambha Pandey, Assistant Professor, Radiation Oncology, Dr. BRAIRCH, AIIMS, New Delhi Dr. Moses Arunsingh, Consultant Clinical Oncologist, Tata Medical Centre, Kolkata Dr. Michelle Norman, Senior Officer (Psycho-oncology Services), Cytecare Cancer Hospitals, Bengaluru Dr. Savita Goswami, Clinical Psycho-Oncologist, Tata Memorial Centre (HBNI), Mumbai Lohithashva, Clinical Nurse Specialist, Pain and Palliative care Department, Cytecare Cancer Hospitals, Bengaluru.
APPENDIX III: EXTERNAL REVIEW COMMITTEE (INTERNATIONAL COMMITTEE)
Dr. Srinivas Raja, Professor, Director of Pain Research, John Hopkins School of Medicine, Maryland, USA Dr. Arun Bhaskar, Consultant Pai Medicine, Imperial College Healthcare NHS Trust, London, UK Dr. Dhanalakshmi Koyyalagunta, Professor, Department of Pain Medicine, The University of Texas M D Anderson Cancer Centre, Houston, Texas, USA Dr. Judith A Paice, Director, Cancer Pain Program Division, Hematology-Oncology, Feinberg School of Medicine, Chicago, USA Dr. Tan Kian Hian, Director and Senior Consultant, Pain Management Centre, Singapore General Hospital, Singapore.
APPENDIX IV: LITERATURE SEARCH
The following terms or MESH terms were used either in combination or single:
“Pain”[Mesh], “Prevalence”[Mesh], “Signs and symptoms”[Mesh], “Syndrome”[Mesh], “Diagnosis”[Mesh], presentation, “Neoplasms”[Mesh], tumours, cancers, physical assessment”, “Pain Measurement”[Mesh], “pain scale”, psychosocial, assessment, “cognitively impaired‘, “psychological distress”, distress, “Emotions”[Mesh] “Nursing”[Mesh], “prime assessor”, “Palliative Care”[Mesh], “supportive care‘‘, “cancer pain management”, “Patient-Centered Care”[Mesh], “Patient Care Team”[Mesh], “Patient Care Management”[Mesh], “Primary Health Care”[Mesh], “Physicians, Family”[Mesh]), interdisciplinary, Education”[Mesh], outcome, barrier, “World Health Organization”[Mesh], “Guideline “[Publication Type], “cancer pain ladder”, “World Health Organization three step analgesic ladder”[Mesh], Drug Therapy”[Mesh], “Analgesics, Opioid”[Mesh], “administration and dosage”[Subheading], titration, “breakthrough pain”, “Drug Tolerance”[Mesh], “Adjuvants, Pharmaceutic”[Mesh], “adjuvant analgesics”, “pregabalin “[Substance Name], “Ketamine”[Mesh], “Dexamethasone”[Mesh], corticosteroid, “opioid rotation”, “opioid switching”, “alternative opioid”, “Bisphosphonates”[Mesh], “Sedation score”, “Morphine protocol”, “Radiotherapy”[Mesh], “Soft Tissue Neoplasms”[Mesh], “Behaviour Therapy”[Mesh], “Cognitive Therapy”[Mesh], “Physical Therapy Modalities”[Mesh], “Acupuncture”[Mesh], “Massage”[Mesh], “Exercise”[Mesh], “Exercise”[Mesh], “Nerve Block”[Mesh], “Injections, Spinal”[Mesh], “intrathecal therapy”, “Vertebroplasty”[Mesh], “follow-up”, “Physician's Role “[Mesh], “community care”, “home program*”, “general practitioner”, hospice, “pain clinic”, “Outpatients”[Mesh], “Outpatient Clinics, Hospital”[Mesh], “Ambulatory Care”[Mesh]
APPENDIX V: CANCER PAIN MANAGEMENT QUESTIONNAIRE
How many patients of cancer pain do you manage per month? What is the most frequent cancer pain that you encounter in your daily practice? What are the clinical presentations of cancer related pain? What are the methods used for clinical assessment of cancer pain? What are the principles of management of pain in patients with cancer? What is the WHO Analgesic Ladder? What are its principles? How effective is it in clinical practice? Do you follow WHO step ladder approach for cancer pain management? What do you prefer for step II and step III of WHO ladder? What non-pharmacological techniques do you use to manage Cancer Pain Do you screen all patients of substance abuse? If yes, which scale do you use. What medications do you use to manage cancer pain What are the major side-effects you observe due to pharmacological management and how do you manage it? What are the adjuvant analgesics in cancer pain management? What are the pharmacological strategies for breakthrough pain and other acute pain crises? What are the roles of anti-cancer therapy in the management of cancer pain? Do you manage patients using Interventional Techniques? If yes, which interventional techniques and in what percentage of patients? What are the relative efficacy and safety of current invasive treatments for the treatment of cancer-related pain? Do you think current treatment guidelines for cancer pain management are sufficient? If no, what changes do you suggest? According to you, what steps need to be taken to spread the awareness regarding cancer pain management?






