Translate this page into:
The Psychometric Properties and Factor Structure of Persian Version of Edmonton Symptom Assessment Scale in Cancer Patients
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Edmonton Symptom Assessment Scale (ESAS) was developed to assess objective and subjective symptoms in patients with cancer in all stages of their disease.
Aim:
The aim of the study was to translate and determine the psychometric properties of ESAS in an Iranian population.
Materials and Methods:
The current study was carried out to determine reliability and validity of ESAS using 246 patients with cancer in Imam Khomeini Hospital, Ardabil, Iran. After translating the instrument to Persian, content and face validity, discriminant validity, internal consistency, and test-retest were done to determine psychometric properties of ESAS. Furthermore, the construct validity was determined using confirmatory factor analysis to evaluate factor structure of the tool in two models: single factor and three factor.
Results:
With regard to goodness of fit indices including comparative fit index, incremental fit index, and normed fit index, factor structure of ESAS was confirmed with one factor and nine items. Because the values of average variance extracted of each dimension were less than the square of correlation coefficients between the three dimensions of ESAS, three-factor model was not confirmed. Discriminant validity was confirmed by finding significant differences between the two groups, patients with good general and critically ill conditions. Cronbach's alpha for the overall ESAS was 0.88, and correlation between test-retest with 4–6 h interval was 0.86 (r = 0.86 P < 0001).
Conclusions:
This study showed that Persian version of ESAS with same factor structure mentioned in the original version is an applicable tool for assessing objective and subjective symptoms in Iranian patients with cancer.
Keywords
Cancer
Edmonton Symptom Assessment Scale
validity
INTRODUCTION
Cancer is a major cause of death in the world. Cancer affected 12.7 and 14.1 million people, in 2008 and 2012, with a mortality rate of 7.6 and 8.2 million people, respectively.[12] In Iran, cancer is the third leading cause of death and its reported incidence rate in men and women is 98 and 110 cases per 100,000 people, respectively. The reported cancer-induced death is 41 and 65 deaths per 100,000 people among men and women, respectively.[3] During the course of the disease and treatment of cancer, patients experience several complications, the most common of which are pain (80%), fatigue (90%), weight loss (80%), appetite loss (80%), nausea (90%), anxiety (25%), shortness of breath (50%), and dizziness (80%).[4] The manifestation of these symptoms impairs patients' quality of life and functionality and can interrupt the process of treatment and reduce the effectiveness of treatment protocols.[5]
Given that many of the annoying symptoms in cancer patients are subjective, it is difficult for nurses to assess and control them. This reveals the importance of having a standard tool that can identify both objective and subjective problems of patients for nurses.[6] Numerous questionnaires have been developed to evaluate physical signs as well as cognitive and mental performance in cancer patients.[789101112] One of such tools is the Edmonton Symptom Assessment Scale (ESAS). It was first designed by Bruera et al. in 1991 to evaluate common problems among patients with cancer.[9] Each ESAS item is scored from 0 to 10. The total symptom distress score ranges from 0 to 100.[13] Due to its ease of use, ESAS was quickly used in palliative care and care programs for cancer patients and was adopted by different researchers in Spain, Italy, Thailand, Germany, Switzerland and China.[131415161718] Dong et al. for instance evaluated the psychometric properties of ESAS in China and approved its criterion validity, internal consistency, and reliability.[18]
One of the issues that have been less addressed in previous studies is the factor structure of ESAS. Although Bruera et al. introduced a one-dimensional structure (total symptom distress score) for ESAS, Carvajal et al. through exploratory factor analysis suggested that a reasonable structure for ESAS is a three-dimensional including soft physical symptoms (nausea, appetite, drowsiness, weakness), emotional components (anxiety, depression, a feeling of well-being, and difficulty in sleeping), and hard physical symptoms (pain and difficulty in breathing).[13] Several studies have evaluated the psychometric properties of ESAS, but they did not address its factor structure.[914151619] This is while further studies appear necessary to examine the factor structure of ESAS.
Palliative care has recently started in Iran,[20] and no studies have yet been conducted to standardize a proper assessment system to assess the cancer patients' symptoms in Iran. ESAS is simple and comprehensive compared to other tools and can be easily completed by patients and also easily interpreted; hence, it can be used in care centers for cancer patients in Iran, provided that its psychometric properties are approved. Therefore, the present study was conducted to determine the psychometric properties of ESAS in Iran and also to examine its factor structure in one-dimensional[9] and three-dimensional models according to the dimensions suggested by Carvajal et al.[13]
MATERIALS AND METHODS
This methodological study was conducted to translate and validate ESAS in cancer patients in the Oncology-Hematology wards and Chemotherapy Clinics of Imam Khomeini Hospital in Ardabil, Iran. The inclusion criteria were aged over 18 years, the ability to communicate verbally and consent to participate in the study. The exclusion criteria were excessive fatigue, psychological diseases (according to the patient's history and medical records), and the inability to answer the questions.
After obtaining permission from the ESAS designers, the tool was translated to Persian by two English experts separately and then compared and corrected to prepare a final version; then, it was retranslated to English. The content and face validity (content validity index [CVI]), discriminant validity, criterion validity, and construct validity (confirmatory factor analysis [CFA] and discriminant validity using statistical analysis), internal consistency, and stability reliability were used to assess the psychometrics of the tool. The CVI was used to determine content validity of the tool according to the views of 13 professors and experts after the translation of ESAS, where the CVI for relevance, simplicity, and clarity of the Persian version of ESAS was 100%, 100%, and 92.3%, respectively.
After a face-to-face discussion and briefing the subjects about the objectives and method of the study, their consent was obtained, and the questionnaire was read and completed by the researcher for them. ESAS was used in the nine items introduced by Bruera et al.,[9] and the tenth item was blank and filled according to the other problems that patients have experienced.
To determine discriminant validity according to the patient's general condition (Karnofsky Performance Scale), the patients were divided into two groups of critically ill patients and patients with a good general condition, and ESAS was completed for each group separately. Finally, the discriminative validity was determined by comparing the scores of the two groups. The Functional Assessment of Cancer Therapy-General (FACT-G) was used as a standard tool to determine criterion validity. The tool was used by Rezaei et al. in 2011 in Iran and its content validity and internal consistency were approved.[21] Various studies have approved the validity of FACT-G as standard criteria for evaluating ESAS.[101622]
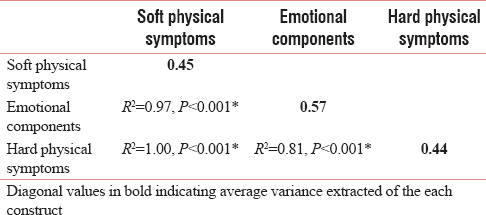
The CFA of ESAS was used to evaluate its factor structure in two models. In the first model, ESAS was considered a single-factor scale with nine items as introduced by Bruera et al.[9] In the second model, based on the model introduced by Carvajal et al.,[13] ESAS was considered a three-dimensional scale including soft physical symptoms (nausea, appetite, drowsiness, weakness), emotional components (anxiety, depression, a feeling of well-being, and difficulty in sleeping), and hard physical symptoms (pain and difficulty in breathing). Since a small number of patients mentioned other symptoms (tenth item), the “difficulty in sleeping” item was not examined in this study. In the next step, discriminant validity of the three-dimensional model was calculated using statistical tests for further analysis of the construct validity. To determine the discriminant validity at this point, it was assumed that despite the correlation of the three factors (dimensions) of the tools, each factor should be separated from other factors so that two or three of them are not considered one factor. For this purpose, the average variance extracted (AVE) was compared to the square of the correlation between factors. Discriminant validity is confirmed when the square of correlations between factors is less than every single average variance extracted.[23242526]
The Cronbach's alpha coefficient was used to evaluate the reliability of internal consistency and to evaluate the stability reliability, a retest was taken from 242 patients within 3–6 h, and the correlations between the two tests were calculated. The CFA was conducted using the LISREL software version 8.8 and other analyses were performed using SPSS for Windows, Version 15.0 (SPSS Inc., Chicago, Illinois, USA).
RESULTS
Data were collected during 6 months from oncology centers and clinics of Imam Khomeini Hospital in Ardabil, Iran. A total number of 260 eligible patients were enrolled. Among them, 14 patients were excluded because of unwillingness to answer all items. Totally, data from 246 patients were analyzed, among whom 120 patients were admitted to hematology-oncology ward and 126 were outpatients in the chemotherapy and radiotherapy clinic. Their mean age was 55.19 ± 14.11 years, ranging from 19 to 84 years. Among the subjects, 137 were men (55.7%), 205 were married (83.3%), 126 were illiterate (51.2%), 102 were homemaker (41.5%), 154 had gastrointestinal cancer (62.6%), and <6 months has passed since the diagnosis in 137 patients (55.7%).
The discriminant validity of the tool was studied by comparing the scores of ESAS in two groups of critically ill patients and patients with a good general condition. Since the scores of ESAS and all items were significantly higher in the group of patients with a good general condition compared to the critically ill patients, the discriminant validity of the tool was confirmed [Table 1].

The FACT-G was used as a standard tool to determine the criterion validity. The Spearman's Rho test showed a high and inverse correlation between ESAS and FACT-G (r = −0.74, P < 0.001). Since higher scores in FACT-G indicate better performances and also lower scores in ESAS indicate less distress in patients; thus, the inverse correlation between these two tools can approve the criterion validity of ESAS. A relatively high and inverse correlation was also found between ESAS and physical well-being (r = −0.81, P < 0.001), emotional well-being (r = −0.39, P < 0.001), and functional well-being (r = −0.53, P < 0.001), while there was no significant relationship between ESAS and family/social well-being dimension (r = −0.08, P = 0.2) in FACT-G.
The construct validity was determined using CFA to evaluate factor structure of the tool in two models: single factor and three factor. The Chi-square/df in the single-factor and three-factor model was 4.25 and 3.14, respectively. According to coefficients of other goodness of fit indices [Table 2 and Figures 1, 2], the goodness of fit was confirmed in both single-factor and three-factor models. As shown in Table 3, the AVE (diagonal value in bold) of each construct is less than the square of correlation coefficients between the respective constructs. It shows that the three dimensions of ESAS are highly correlated with one another and their discriminant validity cannot be statistically confirmed.


- Standardized parameter estimates for Model I (one-factor Edmonton Symptom Assessment Scale). Weak: Weakness, Naus: Nausea, Drows: Drowsiness, Appet: Appetite, Dep: Depression, Anx: Anxiety, WellB: Feeling of well-being, DBreath: Difficulty in breathing

- Standardized parameter estimates for Model II (three-factor Edmonton Symptom Assessment Scale). Soft: Soft Physical Symptoms, Emotion: Emotional Components, Hard: Hard Physical Symptoms Weak: Weakness, Naus: Nausea, Drows: Drowsiness, Appet: Appetite, Dep: Depression, Anx: Anxiety, WellB: Feeling of well-being, DBreath: Difficulty in breathing
The correlation among nine items was analyzed to determine the internal consistency. The total alpha coefficient of the scale was α = 0.88. In addition, the Cronbach's alpha coefficient during the assessment of the internal consistency for the dimensions of soft physical symptoms, emotional components, and hard physical symptoms was 0.74, 0.78, and 0.57, respectively. The correlation between the two administrations of the test with a 3–6 h interval in 242 samples was 0.86 (P < 0.0001, r = 0.86). Assessing the correlation between ESAS dimensions in the test and retest showed that the highest correlation pertained to the loss of appetite (r = 0.85) and the lowest correlation to the feeling of well-being (r = 0.66) and the correlation coefficients of other items were between the two values (r = 0.73–0.82).
DISCUSSION AND CONCLUSIONS
Checking the symptoms of cancer patients is a key element of palliative care. Since ESAS is a simple comprehensive tool that easily specifies acute problems of the patients, the present study was conducted to evaluate the psychometric properties and factor structure of ESAS in patients with cancer. The results of this study showed that the Iranian version of ESAS had a good reliability and validity.
The discriminant validity of ESAS is approved because it had a significantly higher score in critically ill patients compared to the patients with a good general condition (P < 0.001). Previous studies have also reported good discriminant validity for ESAS. Carvajal et al. for instance divided patients into three groups using Karnofsky Performance Scale and found an inverse correlation between Karnofsky score and ESAS items, i.e., an increase in Karnofsky score (improvement in patient performance) decreased ESAS score. They also showed that admitted patients had higher ESAS scores than outpatients, which further approve the discriminant validity of ESAS.[13] Chang et al. (2000) also found an inverse correlation between Karnofsky score (as a better performance measure in patients) and all ESAS items.[10] Although the total score and the scores of ESAS dimensions were different in the two groups of patients, nausea did not show a significant difference. It appears that nausea, regardless of the patient's general condition, was more or less present in all patients and is not a good measure to discriminate between people with good and bad general conditions. Carvajal et al. observed no significant difference in nausea score between the two groups of good and bad general conditions, either.[13]
Based on the high correlation of ESAS and FACT-G, its criterion validity was confirmed. It was also found that ESAS was highly correlated with FACT-G dimensions including physical well-being, emotional well-being, and functional well-being, while there was no relationship between ESAS and the family/social well-being dimension. The poor relationship between ESAS and the family/social well-being in the FACT-G was expected because ESAS items include physical and psychological symptoms but not family or social items. Previous studies in this field have approved criterion validity of ESAS, too.[101319] For example, Chang et al. examined the criterion validity and found a relatively high correlation between ESAS and FACT-G (r = −0.69).[10] Furthermore, Yeşİlbalkan et al. used Rotterdom Symptom Checklist (RSCL) to evaluate criterion validity and found a high correlation between ESAS and RSCL scores (r = 0.75).[19]
The assessment of factor structure of ESAS confirmed original ESAS introduced by Bruera et al. in the form of nine items and one dimension.[9] Previous studies examining the psychometrics of ESAS also confirmed the one-dimensional model.[1013141618] However, despite the confirming psychometric properties of ESAS, Carvajal et al. used exploratory factor analysis in examining the factor structure of the tool and achieved three dimensions of soft physical, hard physical, and emotional components,[13] which was different from the one-dimensional factor structure introduced by Bruera et al. The present study performed CFA and discriminant validity using SEM to confirm or reject the three-dimensional factor structure of ESAS introduced by Carvajal et al.[13] Although the goodness of fit indices of the three-factor structure of ESAS was approved, the high correlation between the dimensions indicates that three dimensions of ESAS overlap to the extent that they cannot be differentiated as separate dimensions. The approved one-dimensional factor structure is also another proof of the rejection of the multidimensionality of ESAS. Meanwhile, it was expected that if multidimensionality of ESAS was proved, the single-factor model was rejected because in such conditions, the tool is potentially made of more than one factor.[232425] In addition, the higher Cronbach's alpha coefficients for single-factor structure (α = 0.88) compared with the three-factor model (α = 0.57–0.78) show that the single-factor structure of ESAS is preferred to the three-factor structure.
The test-retest method indicated a good reliability for ESAS. Previous studies have shown contradictory results in terms of its reliability, which might be due to the differences in the interval between the two tests. For example, Chang et al. (2000) conducted a study in the US to examine the reliability and validity of ESAS and examined the test-retest in two intervals. Their results showed that the retest had a high correlation with the test after 1 day, while it reduced significantly with a 1-week interval.[10] One of the problems of retesting is that the two tests might not have the same conditions. Since ESAS examines symptoms that are mostly physical, the shorter time between the two tests will lead to a higher correlation. For example, in the study of Carvajal et al., performing the test-retest with 4–6 h intervals, the results indicated a very high correlation between the two tests.[13] All in all, according to the results of this study and previous studies,[1016171927] it appears that ESAS has an acceptable reliability.
This study showed that based on content, criterion, and discriminant validities and stability and internal consistency of the Persian version of ESAS, the tool can be used to measure the distressing symptoms of cancer patients in Iran. The results of this study can be a cornerstone to further studies in this field and also a step toward the widespread use of this valuable tool at all medical, rehabilitation, and palliative care centers for cancer patients. One of the features of this study was investigating the factor structure of the tool in two models (one factor and three factor). Although ESAS classification in three dimensions of soft physical, emotional, and hard physical can be more effective in planning and performing nursing care, the results showed that ESAS is more acceptable under the total symptom distress score, and in all patients (patients with good general and critically ill conditions), all nine items emerge or disappear together.
One of the limitations of the study was using FACT-G for criterion validity assessment. Although the tool is used in Iran and its content validity and internal consistency are approved,[21] it is not completely localized in accordance with the standards of psychometrics in Iran and should be cautiously used as a criterion. ESAS can be used by both the assessor and the patient, but it was only completed by researchers in the present study; therefore, it is suggested that in the future studies, the tool be completed by patients, too. Another limitation of this study was conducting the research among cancer patients in Ardabil which makes the generalization of the results more difficult. It is recommended that the psychometric properties of the tool be assessed in other parts of Iran, too.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This article was extracted from a master's thesis approved by Ardabil University of Medical Sciences (ethics code: ARUMS.rec.IR.1395.2). The officials and staff of the Nursing and Midwifery Faculty of the University of Medical Sciences and the Cancer Ward of Imam Khomeini Hospital and patients participating in this study are sincerely thanked.
REFERENCES
- Impact of a palliative care consultation team on cancer-related symptoms in advanced cancer patients referred to an outpatient supportive care clinic. J Pain Symptom Manage. 2011;41:49-56.
- [Google Scholar]
- The symptoms of advanced cancer: Relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer. 2000;8:175-9.
- [Google Scholar]
- Assessing symptom distress in cancer patients: The M.D. Anderson symptom inventory. Cancer. 2000;89:1634-46.
- [Google Scholar]
- Measuring the Quality of Life of Cancer Patients with the Rotterdam Symptom Check List (RSCL): A Manual. Northenrn Centre Healthcare Research (NCH) Netherlands University Groningen 1996
- [Google Scholar]
- The functional assessment of cancer therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570-9.
- [Google Scholar]
- The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6-9.
- [Google Scholar]
- The memorial symptom assessment scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A:1326-36.
- [Google Scholar]
- A comprehensive study of psychometric properties of the Edmonton Symptom Assessment System (ESAS) in Spanish advanced cancer patients. Eur J Cancer. 2011;47:1863-72.
- [Google Scholar]
- Edmonton symptom assessment scale: Italian validation in two palliative care settings. Support Care Cancer. 2006;14:30-7.
- [Google Scholar]
- Reliability and validity of a Thai version of the Edmonton symptom assessment scale (ESAS-Thai) J Pain Symptom Manage. 2011;42:954-60.
- [Google Scholar]
- Validation of the new version of the minimal documentation system (MIDOS) for patients in palliative care: The German version of the Edmonton Symptom Assessment Scale (ESAS) Schmerz. 2010;24:596-604.
- [Google Scholar]
- Symptom assessment in elderly cancer patients receiving palliative care. Crit Rev Oncol Hematol. 2003;47:281-6.
- [Google Scholar]
- Psychometric validation of the Edmonton Symptom Assessment System in Chinese patients. J Pain Symptom Manage 2015. 2015;50:712-17.e2.
- [Google Scholar]
- Validity and reliability of the Edmonton Symptom Assessment Scale in Turkish cancer patients. Turk J Cancer. 2008;38:62-7.
- [Google Scholar]
- Palliative care in Iran: Moving toward the development of palliative care for cancer. Am J Hosp Palliat Care. 2016;33:240-4.
- [Google Scholar]
- Quality of life in gynecologic canter patients before and after chemotherapy. J Babol Univ Med Sci. 2011;13:78-84.
- [Google Scholar]
- Single-vs. multiple-item instruments in the assessment of quality of life in patients with advanced cancer. J Pain Symptom Manage. 2010;39:564-71.
- [Google Scholar]
- Using confirmatory factor analysis to manage discriminant validity issues in social pharmacy research. Int J Clin Pharm. 2016;38:731-7.
- [Google Scholar]
- Product development practices and performance: A structural equation modeling-based multi-group analysis. Int J Prod Econ. 2006;103:286-307.
- [Google Scholar]
- Revisiting the dimensional structure of the Edinburgh postnatal depression scale (EPDS): Empirical evidence for a general factor. BMC Med Res Methodol. 2011;11:93.
- [Google Scholar]
- Evaluating structural equation models with unobservable variables and measurement error. J Mark Res. 1981;18:39-50.
- [Google Scholar]
- Validation of the Edmonton symptom assessment system in Korean patients with cancer. J Pain Symptom Manage. 2013;46:947-56.
- [Google Scholar]






