Translate this page into:
Prognostic Factors of 30-day Survival of Patients with Malignant Pleural Effusion
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Treatment of malignant pleural effusion (MPE) depends on the 1 month prognosis of patients. Until now, there is no study evaluate factors affecting 1 month survival.
Aims:
This study aims to determine the predictors of survival within 1 month.
Methods:
Prospective study of 102 patients with MPE. Biochemistry data of pleural fluid, characteristics of tumor, and massiveness of the effusion were analyzed to determine their effect on 30-day survival of the patients. Univariate analysis was performed using Chi-square. All prognostic factors that had P < 0.25 were included in multivariate analysis using Cox regression.
Results:
Median age of patients was 51 years, most of them were female (56%). Common primary sites of tumor were lung (31%), breast (19%), and lymphatic tissue (11%). In univariate analysis, factors that have P < 0.25 were low glucose concentration in pleural fluid (P = 0.01), high lactate dehydrogenase concentration in pleural fluid (P = 0.25), and high risk tumor (P = 0.24). In multivariate analysis, only low glucose concentration was significantly related to poor survival within 1 month (hazard ratio 2.85 [1.10–7.61], P = 0.03).
Conclusions:
Low level of glucose in pleural fluid is an important factor related to 30-day survival in patients with MPE. It can be used to determine prognosis-based treatment objectively.
Keywords
Malignant pleural effusion
prognostic factor
survival
INTRODUCTION
Malignant pleural effusion (MPE) may decrease survival and quality of life.[1] The main goal of MPE treatment is drainage the excessive volume in the pleural space to relieve symptoms and increase the quality of life of the patients. In general, selection of most appropriate treatment must be individualized.[2] MPE can be treated with conventional or modern technique, depends on the short-term (1 month) prognosis of the patients and the progressivity of the disease.[3] According to British Thoracic Society guideline, patients with life expectancy below 1 month, should be treated by transient pleural aspiration. If the life expectancy is longer, then the patients should be treated by tube thoracostomy or more advanced technique such as pleurodesis and long-term indwelling catheter.[4] Other guideline states that pleurodesis should be planned only if the patient has life expectancy more than 3 months.[5]
Unfortunately, there is no study evaluates factors affecting 1 month survival in patients with MPE until now. This study aims to determine whether 1 month survival is affected by the biochemistry of the pleural fluid, characteristics of tumor, and massiveness of the effusion. Hopefully, following this study, the determination of treatment technique can be more objective.
METHODS
This study was a prospective study, approved by Ethical Committee of Faculty of Medicine University of Indonesia – Cipto Mangunkusumo Hospital (No. of Approval: 99/PT02.FK/ETIK/2012).
Target population of this study was patients with MPE who were hospitalized in Cipto Mangunkusumo Hospital within 2012–2016. The diagnosis of MPE based on physical examination, thorax imaging, and pleural fluid analysis. These findings were confirmed by cytology of pleural fluid. Negative or inconclusive results were confirmed by second cytology examination. Any patients with negative cancer cells or inconclusive results from the second examination were excluded from this study. The demographical, clinical, characteristics of tumor, and pleural fluid analysis data were collected. There were 102 patients included in this study. Patients were followed up for a period of 30 days.
Statistical analysis
Data were analyzed using SPSS program (IBM). In the univariate analysis, pleural fluid value (protein, glucose, lactate dehydrogenase [LDH]), characteristic of tumor (high-risk tumor, metastasis to other organ), and the massiveness of effusion were analyzed using Chi-square. All prognostic factors that had P < 0.25 were included in multivariate analysis using Cox regression. The results will be presented in hazard ratio (HR) and hazard function curve.
Prognostic factors cut-off and definition
High-risk tumor (lung, gastrointestinal, ovary, renal, soft tissue, oral, and prostate) was defined as tumor that had a median survival less than the entire median survival, as stated by Heffner et al.[6] Cutoff the value of protein effusion was 3.85 g/dl, as stated by Bielsa et al.[7] Cutoff value of glucose and LDH effusion was 60 mg/dl and 600 U/L, respectively, as stated by Martínez-Moragón et al.[8] Massive effusion was defined as complete or almost complete opacification on thorax X-ray.
RESULTS
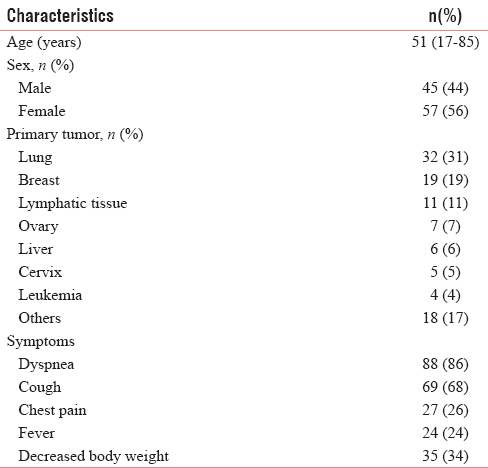
The current study consisted of 102 eligible patients. The characteristics of the study population were outlined in Table 1. The median age of the patients was 51-year-old, and most of them were female (56%). Dyspnea and cough were the main symptoms of the patients. The most common sites for primary tumor were lung (31%), breast (19%), and lymphatic tissue (11%). Other sites for primary tumor (18%) that also found in this study were prostate, thyroid, oropharynx, bladder, and musculoskeletal.
Results of univariate analysis were described in Table 2. Biochemical parameter of pleural fluid showed that only glucose and LDH that had P < 0.25. Patients with higher glucose levels and lower LDH levels had better survival. Interestingly, the patients with high-risk tumor had longer survival, with P value below 0.25. The massiveness of effusion did not influence 30-day survival of the patients significantly.
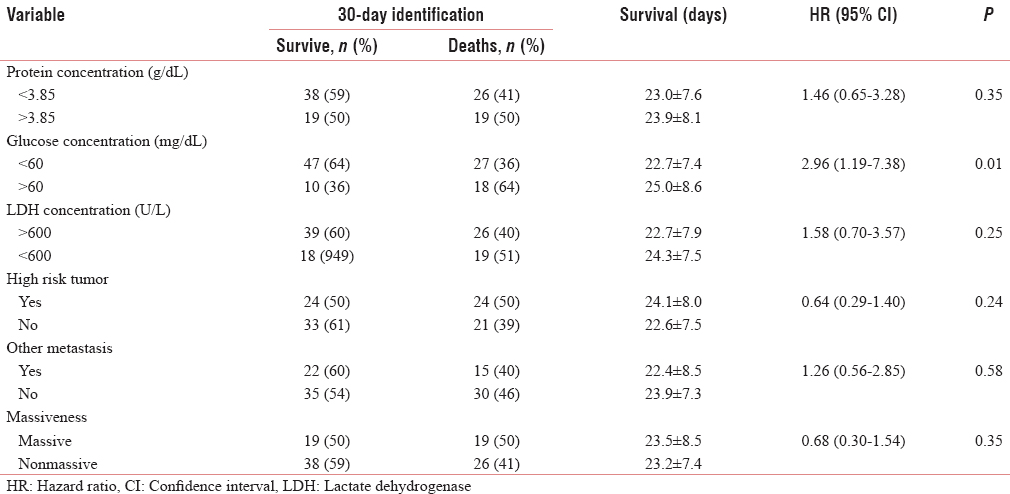
Among the prognostic factors, only glucose concentration, LDH concentration, and the presence of high-risk tumor were evaluated into multivariate stepwise logistic regression analysis. Based on the analysis [Table 3], only low concentration of glucose in pleural fluid was found as the predictor of 30-day survival (HR = 2.85, P = 0.03). The cumulative hazard of low glucose concentration to 30-day-survival was presented in Figure 1.

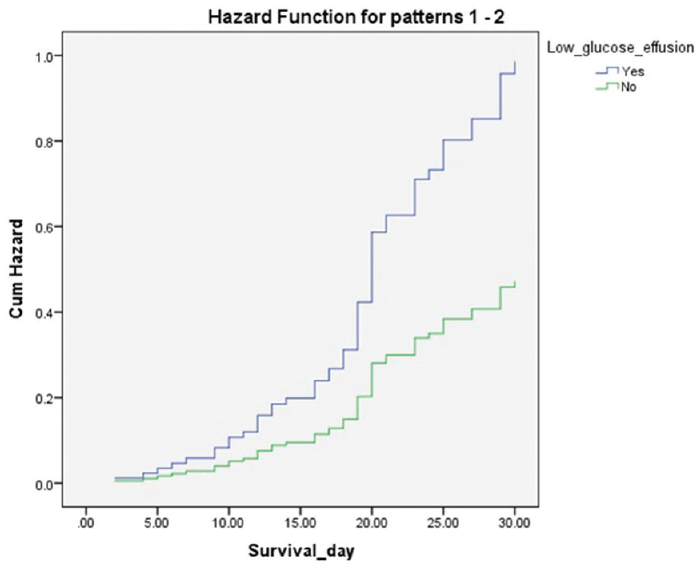
- Cumulative hazard of low concentration of glucose to 30 day-survival
DISCUSSION
The finding of tumor cells in pleural fluid indicates advanced stage of the disease. It causes poor survival and quality of life. Heffner et al. reported that 1 month survival of patients with MPE was 80.3%.[6] However, 1 month survival in our study only 55.9%. The median age of patients in our study was 51 years. It was different from other studies that show median age of patients with MPE usually older than 60 years.[910] The distribution of sex in our study did not different from other study.[10] The survival in patients with MPE was not associated with age and gender.[11] Lung and breast cancer predominated as the primary site of tumor, as found in other study.[6712]
Low glucose concentration in pleural fluid was known to be associated with poor survival, although different cutoff values had been reported. Martínez-Moragón et al. found that concentration of pleural fluid glucose <60 mg/dl was a good predictor of lower survival (5 months vs. 11 months, P < 0.01).[8] This relationship could be explained because an abnormal pleural membrane (tumor or fibrosis) impaired glucose transfer from blood to pleural fluid across pleural membrane.[13] In our multivariate analysis, low glucose concentration was the only significant predictor of poor survival within 1 month (HR 2.85 [1.10–7.61], P = 0.03).
The concentration of LDH in pleural fluid was known to be associated with prognosis of the patients. High LDH level worsened the survival of patients. Martínez-Moragón et al. reported that LDH concentration >600 U/L was a significant predictor of poor survival (6 months vs. 10 months, P < 0.01).[8] Other multivariate study also found a mean survival of 2.9 months if LDH concentration >560 U/L.[7] However, in our multivariate study, high LDH concentration insignificantly related to 30-day survival of the patients.
Bielsa et al. reported a mean survival of 2.2 months if protein concentration in pleural fluid <3.85 g/dl.[7] Low concentration of protein was associated with a lower survival due to plasmatic hypoproteinemia accompanying an advanced emaciation stage. Contrarily, low protein concentration was not significantly related to 30-day survival in our study.
Many studies reported that patients with breast cancer, mesothelioma, and lymphoma had longer survival than patients with lung, ovary, and gastrointestinal cancer.[678] Heffner et al. grouped tumors with median survival <4 months (median survival of entire MPE patients) as high-risk tumors. It consists of cancers of the lung, gastrointestinal, ovary, renal, soft tissue, oral, and prostate.[6] Interestingly, we found that patients with high-risk tumor had better 30-day survival. It can be possibly explained because patients with high-risk tumor usually get more comprehensive treatment, such as hemodialysis for renal impairment or more potent antibiotic for lung cancer with infections. Obviously, it needs further exploration or subgroup analysis to answer this problem.
Most common cause of massive pleural effusion was malignancy (53.6%). Massive MPE was related with the extent of the metastasis in pleura. Patients with nonmassive MPE had a significantly better survival than those with massive MPE (8 months vs. 5 months, P < 0.01).[14] However, our study found that this massiveness was not related with prognosis within 1 month.
CONCLUSIONS
Low level of glucose in pleural fluid is an important prognostic factor related to 30-day survival in patients with MPE (HR = 2.85). It can be used to determine prognosis-based treatment objectively.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Malignant pleural effusion: Tumor-host interactions unleashed. Am J Respir Crit Care Med. 2012;186:487-92.
- [Google Scholar]
- Management of malignant pleural effusion: Options and recommended approaches. Thorac Cancer. 2013;4:9-13.
- [Google Scholar]
- Management of a malignant pleural effusion: British thoracic society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii32-40.
- [Google Scholar]
- An update in the management of malignant pleural effusion. Indian J Palliat Care. 2011;17:98-103.
- [Google Scholar]
- Pleural fluid pH as a predictor of survival for patients with malignant pleural effusions. Chest. 2000;117:79-86.
- [Google Scholar]
- Prognostic significance of pleural fluid data in patients with malignant effusion. Eur J Intern Med. 2008;19:334-9.
- [Google Scholar]
- Malignant pleural effusion: Prognostic factors for survival and response to chemical pleurodesis in a series of 120 cases. Respiration. 1998;65:108-13.
- [Google Scholar]
- Prognostic factors in patients presenting with pleural effusion revealing malignancy. Respiration. 2014;87:311-6.
- [Google Scholar]
- Important prognostic factors for survival in patients with malignant pleural effusion. BMC Pulm Med. 2015;15:29.
- [Google Scholar]
- Predicting survival in patients with recurrent symptomatic malignant pleural effusions: An assessment of the prognostic values of physiologic, morphologic, and quality of life measures of extent of disease. Chest. 2000;117:73-8.
- [Google Scholar]
- Prognostic factors in patiehnts with malignant pleural effusion: Is it possible to predict mortality in patients with good performance status? J Surg Oncol. 2016;113:570-4.
- [Google Scholar]
- The pathogenesis of low glucose, low pH malignant effusions. Am Rev Respir Dis. 1985;131:737-41.
- [Google Scholar]
- Etiology and prognostic significance of massive pleural effusions. Respir Med. 2005;99:1183-7.
- [Google Scholar]






