Translate this page into:
The Use of the Chuang's Prognostic Scale to Predict the Survival of Metastatic Colorectal Cancer Patients Receiving Palliative Systemic Anticancer Therapy
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
With the increasing number of agents active against cancer, advanced cancer patients including metastatic colorectal cancer (mCRC) patients may continue receiving palliative systemic anticancer therapy (PSAT) near the end-of-life. Validated palliative prognostic models, such as the Chuang's prognostic scale (CPS), may be helpful in identifying mCRC patients with limited survival who are unlikely to benefit from PSAT.
Aim:
To test the ability of the CPS to predict the survival of mCRC under treatment with PSAT.
Methods:
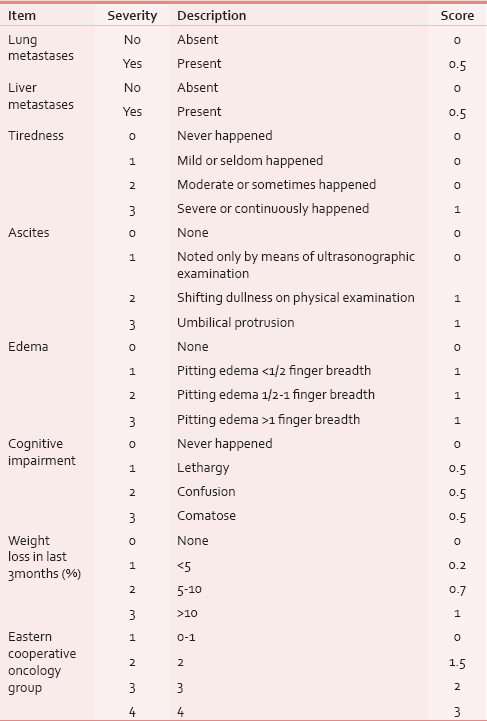
CPS was prospectively assessed in 36 mCRC patients who were receiving PSAT. The scale is based on eight items: ascites, edema, cognitive impairment, liver and lung metastases, performance status, tiredness, and weight loss. The total CPS score ranges from 0 to 8.5 with the higher score indicating worse prognosis.
Results:
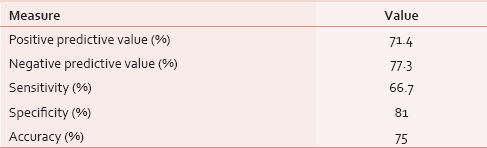
Patients were divided into two groups using a CPS cutoff score of 5, Group 1 with a CPS score ≤5 and Group 2 with a CPS score >5. Using this cutoff value, 3-month mortality was predicted with a positive predictive value of 71%, a negative predictive value of 77%, a sensitivity of 67%, a specificity of 81% and an overall accuracy of 75%. Group 1 patients had a longer median survival of 149 days (95% confidence interval [CI]: 82-216) in comparison to Group 2 patients who had a median survival of 61 days (95% CI: 35-87). The difference in survival was statistically significant (P = 0.01).
Conclusion:
CPS may be useful in identifying mCRC patients with limited survival who are unlikely to benefit from PSAT.
Keywords
Chuang's prognostic scale
End-of-life care
Metastatic colorectal cancer
Palliative systemic anticancer therapy
Prognosis
INTRODUCTION
Chemotherapy administration near the end-of-life (EoL) of patients with incurable cancer indicates aggressive EoL care.[1] Although recognized as an aggressive intervention at the EoL, a number of reports described an increasing number of dying cancer patients receiving chemotherapy close to death.[23] In addition to the direct negative impact that may result from over treatment with chemotherapy at the EoL, it is associated with other forms of aggressive EoL care. Patients who receive palliative chemotherapy near the EoL are more likely to undergo cardiopulmonary resuscitation and/or intubation and to die in intensive care units.[4]
In 2012, colorectal cancer was the 3rd most commonly diagnosed cancer (1.36 million new cases) and the 4th most common cause of cancer death (694,000 deaths) worldwide.[5] Colorectal cancer patients, such as other patients with relatively chemosensitive tumors, may be at a higher risk of receiving chemotherapy at the EoL.[6] There is an increasing number of agents active against metastatic colorectal cancer (mCRC).[7] With these increasing therapeutic choices, it will not be surprising that incurable colorectal cancer patients would continue receiving systemic treatment at the EoL. The use of targeted therapies at the EoL is increasing, and they are used as often as conventional chemotherapeutic agents.[89]
Finding tools to predict survival of patients with incurable colorectal cancer may help in selecting patients who may benefit from palliative systemic anticancer therapy (PSAT). This may avoid terminally ill cancer patients unnecessary aggressive treatment.
A number of prognostic models were developed for the purpose of predicting survival in patients with terminal cancer like the palliative prognostic score, the palliative prognostic index and the Chuang's prognostic scale (CPS).[1011] The use of Palliative prognostic models to aid in treatment decision making had been suggested.[12]
CPS was designed more than a decade ago by Chuang et al. in Taiwan to predict short-term survival in patients with terminal cancer.[10] It was developed in a training set of 356 terminal cancer patients and further validated in a testing set of 184 patients.
We hypothesized that a Palliative prognostic model like CPS might be useful in predicting the survival of mCRC patients receiving PSAT.
METHODS
A prospective cohort study was conducted to investigate the prognostic value of the CPS in a heterogeneous group of 117 patients with incurable gastrointestinal malignancies. This report describes a subgroup analysis that included mCRC patients who were receiving PSAT. Included patients were adults (>18 years) who had histopathological colorectal cancer diagnosis with evidence of distant metastases, and their plan of treatment was PSAT of which they received at least one cycle.
CPS includes eight items: Ascites, edema, cognitive impairment, liver and lung metastases, Eastern Cooperative Oncology Group performance status, tiredness, and weight loss. The CPS items were assessed and scored according to the developers of the tool as illustrated in Table 1.[10] The total CPS score ranges from 0 (all variables are not altered) to 8.5 (all variables are maximally altered).
A receiver operating characteristic (ROC) curve was used to determine the best CPS cutoff value for the prediction of 3-month mortality.
Patients were followed up until death or for at least 3 months. Survival was calculated from the date of CPS assessment until death or last follow-up. The Kaplan-Meier method was used to estimate survival and the log-rank method was used to test for the significance of difference in survival between the CPS groups.
Statistical analysis was performed using the Package for Social Sciences (SPSS) for Windows, version 14 (SPSS Inc., Chicago, Illinois, USA). A P < 0.05 was considered statistically significant.
RESULTS
CPS was assessed in 36 patients with mCRC who were receiving PSAT and were followed up for at least 3 months or until death.
The characteristics of patients are shown in Table 2. The most common prescribed PSAT regimen at the time of CPS assessment was FOLFIRI (folinic acid + fluorouracil + irinotecan) which was prescribed for 21 patients (58%).

Using the ROC curve analysis, the best CPS cutoff value for the prediction of 3-month mortality was 5 [Figure 1].

- The receiver-operating characteristic curve for the prediction of 3-month mortality using the Chuang's prognostic scale
According to this cutoff value, patients were divided into two groups, Group 1 with a CPS score ≤5 (22 [61%] patients) and Group 2 with a CPS score >5 (14 [38.9%] patients). The percentage of patients who survived < 3 months in Group 1 was 23% and in Group 2 was 71% (P = 0.004). The accuracy of a CPS cutoff value of 5 in predicting 3-month mortality is shown in Table 3.
The estimated median survival of whole group of patients was 103 days (95% confidence interval [CI]: 74-132). The median CPS score was 4.35 (range: 2-8). The estimated median survival was 149 days (95% CI: 82-216) for Group 1 and 61 days (95% CI: 35-87) for Group 2. The difference in survival was statistically significant (P = 0.01). The Kaplan-Meier survival curves for the two groups are shown in Figure 2.

- Kaplan–Meier survival curves of patients with metastatic colorectal cancer receiving chemotherapy according to the Chuang's prognostic scale score (Group 1 [≤5] vs. Group 2 [>5])
DISCUSSION
With the advancement of cancer research, there are an increasing number of novel methods to predict prognosis in patients with mCRC like using molecular profiling.[7] However, these methods may not be applicable in many settings, especially when resources are limited. The availability of simple noncostly predictive tools to predict prognosis in this group of patients may facilitate communication with patients and help in decision making.
The CPS is a simple prognostic model that is based on the presence or absence of liver and lung metastases and other simple clinical findings. It was developed to predict short-term survival in a population of terminal cancer patients with an overall median survival of only 13 days.[10] The developers of the CPS used a cutoff score <3.5 to predict 2-week survival with an accuracy of 72% and a cutoff score of <6 to predict 1-week survival with an accuracy of 72% in the training set.
Another study validated the CPS in a different setting and showed that it is useful in predicting the prognosis of advanced cancer patients with relatively longer survival.[13] The median survival of patients included in this study was more than 3 months (103 days). This confirms that CPS may have a prognostic predictive value in advanced cancer patients who are not terminally ill and have an estimated survival of months.
In addition to its use to predict the survival of advanced cancer patients,[1013] CPS was found to be useful in predicting their hospitalization outcome.[13] The current study further suggests another application for the CPS. mCRC patients receiving PSAT with a CPS score >5 had a significantly shorter survival of only 2 months. Furthermore, using the same cutoff point predicted 3-month mortality with an overall accuracy of 74% in this group of patients. This may be of help in decision making when PSAT is considered for mCRC patients. It is unlikely to attain the desirable effect of PSAT when the estimated survival of advanced cancer patients is only 2 months.[14] Accordingly, the CPS may be useful in dividing mCRC patients into two groups. mCRC patients with a score ≤5 (estimated survival of 5 months) may benefit from PSAT administration. For the other group of patients with a score >5 (estimated survival of 2 months), a supportive and palliative plan of care would be more realistic.
This is the first study to investigate the prognosis predictive value of the CPS in advanced cancer patients receiving PSAT. Another Palliative prognostic model, the Palliative prognostic score was used to predict the survival of advanced cancer patients receiving systemic anticancer therapy.[1415] The Palliative prognostic score ranges from 0 to 17.5 and is based on six items, dyspnea, anorexia, Karnofsky performance status, clinician prediction of survival, total white blood cell count and lymphocyte percentage.[16] In one study, a heterogenous population of 173 patients with pretreated advanced solid tumors were grouped according to the Palliative prognostic score before starting further palliative chemotherapy. The group of patients with higher (worse prognosis) Palliative prognostic score had a significantly shorter survival.[14] In another study, the Palliative prognostic score was used to divide 44 patients with nonresectable stomach cancer receiving chemotherapy into three groups with highly significant difference in survival.[15] The results of these two studies assessing the Palliative prognostic score prognostic value, and our results are encouraging and suggest that validated Palliative prognostic models may be helpful in selecting patients with relatively longer survival for whom PSAT may be of benefit. At the same time, this may decrease the aggressiveness of EoL care by reducing PSAT administration for terminally-ill cancer patients. PSAT at the EoL of cancer patients raises many ethical concerns and may contradict the principles of medical ethics. With the lack of positive impact on survival and quality of life of terminal cancer patients, “beneficence” is not achieved. This is the same for “nonmaleficence” when PSAT results in toxicities and distress. Furthermore, the exhaustion of scarce resources to deliver PSAT to dying cancer patients may violate the principle of “justice.” This is especially true in resource-limited settings where there are cancer care priorities such as prevention, early detection, and palliative care.
The limitations of the study included being from a single center and the relatively small sample size. In addition, CPS was not compared to other known prognostic factors in mCRC.
CONCLUSION
CPS may be useful in predicting the survival of mCRC patients receiving PSAT. Consequently, it may be helpful in decision making and discussions related to commencing or withholding PSAT in mCRC patients. This is of particular relevance in resource-limited settings like ours. Further studies are needed to explore the role of the CPS and other Palliative prognostic models in predicting the survival of incurable cancer patients for whom the plan of care is to administer PSAT.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Quality indicators for care of cancer patients in their last days of life: Literature update and experts’ evaluation. J Palliat Med. 2012;15:308-16.
- [Google Scholar]
- Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol. 2011;29:1587-91.
- [Google Scholar]
- Trends in receiving chemotherapy for advanced cancer patients at the end of life. BMC Palliat Care. 2015;14:4.
- [Google Scholar]
- Associations between palliative chemotherapy and adult cancer patients’ end of life care and place of death: Prospective cohort study. BMJ. 2014;348:g1219.
- [Google Scholar]
- Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86.
- [Google Scholar]
- Use of chemotherapy at end of life in oncology patients. Ann Oncol. 2009;20:1555-9.
- [Google Scholar]
- Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med. 2015;66:83-95.
- [Google Scholar]
- Targeted therapy at the end of life in advanced cancer patients. J Palliat Med. 2012;15:991-7.
- [Google Scholar]
- Targeted agent use in cancer patients at the end of life. J Pain Symptom Manage. 2013;46:1-8.
- [Google Scholar]
- Prediction of survival in terminal cancer patients in Taiwan: Constructing a prognostic scale. J Pain Symptom Manage. 2004;28:115-22.
- [Google Scholar]
- Predicting life expectancy in patients with advanced incurable cancer: A review. J Support Oncol. 2013;11:68-74.
- [Google Scholar]
- Prognostic factors in advanced cancer patients: Evidence-based clinical recommendations – A study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23:6240-8.
- [Google Scholar]
- Prediction of in-hospital mortality of patients with advanced cancer using the Chuang prognostic score. Am J Hosp Palliat Care. 2013;30:707-11.
- [Google Scholar]
- The palliative prognostic score and survival in patients with advanced solid tumors receiving chemotherapy. Support Care Cancer. 2008;16:359-70.
- [Google Scholar]
- Usefulness of palliative prognostic score in the treatment of patients with non-resectable gastric cancer. Mol Clin Oncol. 2013;1:253-6.
- [Google Scholar]
- A new palliative prognostic score: A first step for the staging of terminally ill cancer patients. Italian Multicenter and Study Group on Palliative Care. J Pain Symptom Manage. 1999;17:231-9.
- [Google Scholar]






