Translate this page into:
Lofexidine for Treating Opioid Withdrawal Syndrome in Palliative Care Patients
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Patients with advanced malignancy or chronic pain of any etiology are prescribed round-the-clock opioids for effective pain relief. This could be tablets, injections, or transdermal patches. The tolerability profile of these opioid medications varies from patient to patient. Route of administration or the type of opioid medications (for example, tramadol changed to morphine tablets) are sometimes changed by the physician either due to intolerable side effects, due to lesser side effects of a new drug, or for the sake of ease of administration (for example, transdermal patches). Sometimes, patients are deprived of the regular dose for an extended period either due to nonavailability or financial reasons. This change of medication, delay in a scheduled dose, or absence of an opioid can precipitate opioid withdrawal syndrome (OWS) in palliative care patients.
The incidence of opioid withdrawal is approximately 10% in cancer pain patients which is usually manifested when per oral morphine is changed over to transdermal fentanyl. Rescue dose of the original opioid has been shown to be beneficial in such situations to avoid OWS.[1] OWS manifests as papillary dilatation, severe muscle cramps, profuse diarrhea, abdominal cramps, yawning, piloerection, rhinorrhea, lacrimation, hypertension, tachycardia, temperature dysregulation, and at times delirium.[2]
Theoretically, OWS can be managed by providing the regular dose of ongoing medication. This may not be feasible if the original plan was to change the ongoing medication. Alpha-2 agonists such as clonidine and dexmedetomidine have been used to manage OWS and alcohol withdrawal syndrome, but the use is off-label and without United States-Food and Drug Administration (US-FDA) approval.[3] Drugs such as buprenorphine and methadone in tapered doses have been used with variable results for managing OWS.[4]
Lofexidine is an orally available imidazoline adrenergic alpha-2-receptor agonist approved by the US-FDA for managing OWS [Figure 1]. The drug has been approved for a treatment duration of 2 weeks.[5] The chemical structure of lofexidine resembles clonidine which is another centrally acting alpha-2 receptor agonist with some alteration of chemical bond at one level. Lofexidine has high affinity for 2A receptor subtypes, resulting in less antihypertensive activity. By central alpha-2 receptor agonist, the drug reduces the intensity of OWS by reducing norepinephrine levels and also leads to sympatholysis. It leads to clinically less serious bradycardia and hypotension when compared to clonidine and is short acting. It should be used carefully or avoided with concomitant antihypertensives and sedatives such as benzodiazepines. The recommended dose of treating OWS is three tablets of 0.18 mg lofexidine four times a day for 14 days.
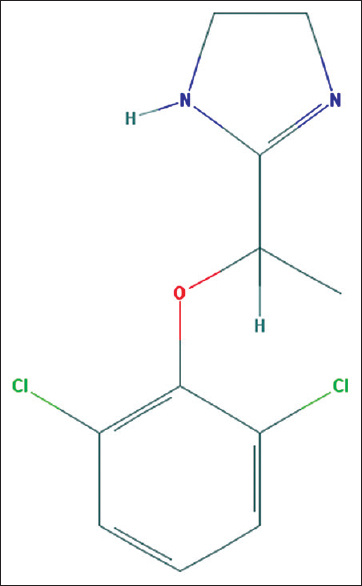
- Chemical structure of lofexidine (Image source: National Center for Biotechnology Information. PubChem Compound Database; CID = 30668, https://pubchem.ncbi.nlm.nih.gov/compound/30668; accessed May 26, 2018)
QTc prolongation is a serious event possible with the use of lofexidine; therefore, it should be used carefully or avoided in susceptible situations (electrolyte imbalances, other drugs that prolong QTc like antiemetics). Dose adjustment is suggested in renal and hepatic impairment. Till date, it has not been used in pregnancy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Opioid withdrawal syndrome on switching from hydromorphone to alfentanil. Palliat Med. 2008;22:191-2.
- [Google Scholar]
- Opioid withdrawal presenting as delirium and role of buprenorphine: A case series. Indian J Psychol Med. 2017;39:665-7.
- [Google Scholar]
- Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2016;5:CD002024.
- [Google Scholar]
- Low dose intramuscular methadone for acute mild to moderate opioid withdrawal syndrome. Am J Emerg Med 2018:pii: S0735-6757(18)30153-0.
- [Google Scholar]





