Translate this page into:
Validation of the EuroQol Five-dimensions - Three-Level Quality of Life Instrument in a Classical Indian Language (Odia) and Its Use to Assess Quality of Life and Health Status of Cancer Patients in Eastern India
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Objectives:
The EuroQol five-dimensions – 3-level (EQ5D) is a versatile quality of life (QOL) instrument with five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) and a visual analog scale. It can be used to calculate quality-adjusted life years. We aimed to evaluate the validity, reliability, and responsiveness of an Odia version of EQ5D and to study the QOL of cancer patients in our part of the country as cancer treatment in India still focuses largely on longevity due to scarcity of resources.
Materials and Methods:
The EQ5D tool was translated into Odia language in collaboration with the EQ group. This tool and the World Health Organization-5 (WHO-5) questionnaires were administered to 155 surgical outpatients and 150 cancer patients in two hospitals of Eastern India. The convergent and discriminant validities (construct validity), concurrent validity, reliability (test-retest method of administering the tool to a part of the population after 7–14 days), and the internal consistency (Cronbach's alpha) were measured using preestablished hypotheses. The data from the cancer patients were analyzed separately.
Results:
The QOL worsened with age and was worse in cancer patients proved that the tool had good construct validity. The Anxiety Depression dimension had good correlation with all the dimensions WHO-5 (rho > 0.4) indicating a good concurrent validity. Internal consistency and reliability of the tool were good (Cronbach's alpha > 0.7). Cancer patients had a poor QOL (mean EQ5D index 0.37SD 0.4) with male patients, patients with Grade II cancer or referred for pain care services and those with living spouses reporting worse QOL.
Conclusions:
The Odia version of the EQ5D has good reliability and validity for the measurement of health status in cancer and outpatient department patients. Cancer patients in this part of the country have a poor QOL and may need a closer look at pain management and improved societal support systems.
Keywords
Cancer
EuroQol five-dimensions
Indian language Odia
Quality of life
Reliability
Validation
INTRODUCTION
The concept of health and wellbeing is a subjective one. The patient's own perspective and assessment of his or her quality of life (QOL) are an important parameter for assessing health outcomes, efficacy, and economic impact of interventions.[1] This requires the use of validated and reliable self-reported questionnaires. The application of any patient-reported questionnaire to a new population requires revalidation of the instrument in the population concerned.[2]
The EuroQol five-dimension (EQ5D) is a generic, preference-based measure of health. It allows for the calculation of quality adjusted life years by adopting preference-based index scores derived from it. This aids decision-making in health care with its varied applications.[34] It is one of the most frequently used instruments for assessing QOL and has been validated in many languages and disease states.[567] Cancer is one of the most frequent disease-specific applications of the EQ5D and its validity and reliability in assessing QOL in cancer patients has been established.[7]
Cancer care in India still focuses largely on longevity. Resources are scarce: The challenge of interpreting health outcomes in terms of psychological morbidity and QOL is recognized, it is often overlooked.[89] A large population in the Eastern part of India are conversant in Odia language. There are no accepted instruments of QOL assessment that have been validated in Odia. We aimed to validate Odia translation of the EQ5D and assess its psychometric properties in cancer patients in Eastern India.
MATERIALS AND METHODS
Patient sample
The sample consisted of 155 patients attending the general surgical outpatient department (OPD) and 150 admitted cancer patients (with all sites and stages of cancer) of a University Teaching Hospital and a Regional Cancer Centre in Eastern India respectively. All adult patients conversant with Odia language and consenting to participate in the study were administered the questionnaires in a face to face interview in both the hospitals by doctors trained in the process: Demographic information was also collected. The study has received permission from the Ethical Committee Review Boards at both the hospitals.
Instruments
EuroQol five-dimension
The EQ5D is a brief self-reported generic measure of current health: A simple two-page questionnaire which has five descriptive questions which may have one of three-level answers and a visual analog scale (VAS) on which patients can mark their current health state. The 5D (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) have three levels of functioning each (no problems, some problems, and unable to/extreme problems). The VAS is a scale from 0 (worst imaginable health state) to 100 (best imaginable health state). Various studies have shown the reliability of the EQ5D in general population and in different patient population including cancer patients.[101112] As the data for the general Indian population are not available, dataset of UK population was used to calculate the EQ5D index values as advised by the EQ group.
World Health Organization-5
WHO-5 Is also a simple worded questionnaire capturing well-being. Developed from the World Health Organization-Ten Well-Being Index, it was conceptualized as a unidimensional measure containing five positively worded items: “I have felt cheerful and in good spirits;” “I have felt calm and relaxed;”“I have felt active and vigorous;” “I woke up feeling fresh and rested;” and “My daily life has been filled with things that interest me.” The most relevant is to be marked on a six-point Likert scale ranging from 0 (not present) to 5 (constantly present).[13] A sum-score can be calculated as a summary measure. Similar to our study, it has previously been used in assessing the convergent validity of the English language version of the EQ5D.[14] The WHO-5 has previously been validated in the Indian population and found to have a high reliability (k = 0.71).[15]
Translation and official approval of EuroQol group
The principal investigator contacted the EQ group for all relevant information regarding the official procedure for translation and approval of the English version of the EQ5D. A constant communication between the members of the translation review team (constituting Odia linguists and study investigators) and the EQ group allowed them to provide feedback and comments on the reports of each stage of translation of the questionnaire [Figure 1].
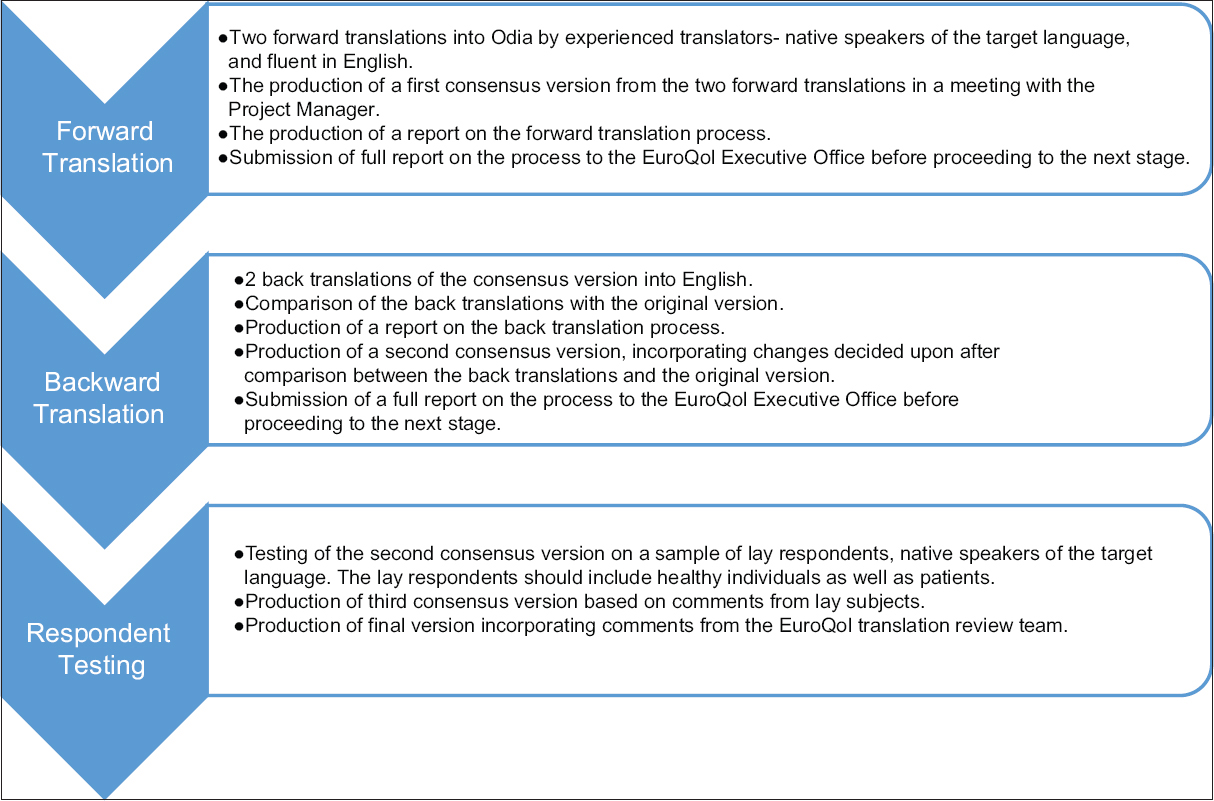
- Steps for translation official approval of Odia version of EuroQol five-dimensions
The aim is to produce an easy and natural-sounding translation which is acceptable to respondents in the target language while also transmitting the meaning of the original. The translation teams were required to include at least one nontechnical person, and the respondent testing was to be done on a spectrum of healthy and sick patients of varied education level. Details of all translators and respondents were required to be submitted to the EQ Group for approval. Official approval was based on the quality and detail of the reports provided to the EuroQol Translation Committee.
Construct validity
Convergent
Convergent and discriminant validity are regarded as subsets of the construct validity of an instrument. Convergent validity tests that constructs that are expected to be related are related. Discriminant (or divergent) validity tests that construct that should have no relationship, do not.[16] To test for convergent validity, the hypothesis made was that there would exist a strong correlation between increasing age and self-reporting of problems in the domains mobility, self-care and usual activities (hypothesis 1). It was also hypothesized that anxiety and worries would be greater among women respondents (hypothesis 2).
Discriminant validity
To test for discriminant validity, it was hypothesized that the EQ5D would be able to distinguish between general surgery OPD patient and cancer patients (hypothesis 3).
Concurrent validity
Concurrent validity between the EQ5D dimensions and the WHO-5 items was assessed using the Spearman rank order coefficients (Spearman's rho). It was hypothesized that there would be a significant correlation between all WHO-5 items and the anxiety/depression domain of EQ5D (hypothesis 4).
Reliability
An instrument or tool is considered reliable if its results are consistent and reproducible. The test-retest measure of reliability was evaluated by re-administering the questionnaire over phone to 15% of the subjects after a gap of 14–21 days.
Internal consistency
This measures the degree of correlation between different items within a tool-items which propose to measure the same construct, should have similar values. It was assessed using Cronbach's alpha, with a value >0.7 being acceptable.
Statistical analysis
The Chi-square test, Mann–Whitney test, or Spearman's correlation coefficient were used where appropriate to test the hypotheses. Strong, moderate, and weak correlations were defined as >0.60, 0.30–0.60, and <0.30, respectively.[17] Intra-class correlation coefficient was used to measure test-retest reliability of the EQ5D responses; a desired value was >0.5.[17] The internal consistency of the scale was calculated with Cronbach's alpha coefficient (minimum acceptable value for alpha was 0.7). A P < 0.05 was considered as statistically significant. Data were analyzed with SPSS for windows (version 21, SPSS Inc., IL, USA).
RESULTS
Patient characteristics
A total of 305 patients were interviewed in between February and August 2014. Of these, 150 patients were inpatients in a dedicated cancer center and 155 were patients who had come to the surgical OPD of a university hospital. Their demography and characteristics are summarized in Table 1. The median age of all the patients was 45 years (range 18–85), the male:female ratio was 1.4:1. Forty percentages of the patients were uneducated, and 23% were graduates with the surgical OPD patients being better educated. More than 76% of all the patients were married and were living with their spouse. Among the patients who attended the surgical OPD, complaints varied widely from skin and soft tissue ailments in 15%, fistula and hemorrhoids 12%, renal and genitourinary complaints in 22%, and gastritis and constipation in 11%. Table 1 lists the relevant details of the cancer patients’ disease.

EuroQol five-dimensions data
The response rate was more than 99% with five missing data for all variables. VAS index had a median value of 0.63 (skewness-0.6), with a range from −0.07 to 1 [Figures 2 and 3]. The calculated utility score had a median value of 0.65 (skewness –1.13), with range from 0.65 to a maximum of 1.00 (best health scenario).

- Histogram of EuroQol five-dimension visual analog scale scores in cancer patients

- Histogram of EuroQol five-dimension visual analog scale scores in outpatient department patients
Test-retest reliability
A total of 15% (45) of patients were called after 2 weeks (median 16 days) of the original interview. The correlation coefficient (Pearsons) for the first and second interviews for EQ5D VAS score and among the cancer patients and OPD patients was 0.72 and 0.55 (strong correlation, P < 0.005), respectively.
Internal consistency
The Cronbach's alpha for the instrument was 0.76 signifying a good internal consistency.
Validity
Construct validity
Convergent validity (H1 and H2)
As hypothesized, with an increase in age, respondents demonstrated a significant increase in self-reported problems in the mobility, self-care and activities dimensions: Reported problems in anxiety and pain did not differ significantly across age [Table 2]. Men reported significantly more problems in the mobility dimension of EQ5D. There was no difference in any other dimensions among men and women [Table 3].


Discriminant validity (H3)
A significant difference was noted in the frequency of reported problems among the surgical OPD and cancer patients in all the dimensions of EQ5D. There was a statistically significant difference in the calculated utility indices of VAS and EQ5D dimensions, with cancer patients reporting a worse QOL in both scores [Table 4].

Concurrent validity
Patients were grouped according to their response on EQ5D and WHO-5. All Spearman rank order coefficients for comparison of EQ5D with the five WHO-5 items were significant (P < 0.05). EQ5 (anxiety) domain of EQ5D had strong negative correlation with all WHO-5 domains (rho = 0.37, 0.43, 0.37, 0.42, and 0.47) as expected. Correlation (negative) was moderately strong (0.3–0.34) between mobility, self-care, and usual activities with feeling active and vigorous
Analysis of patients having cancer
The distribution of disease was as follows-gastrointestinal malignancy in 49, genitourinary in 31, Head, Neck, and Breast cancers in 24 each and lungs cancer in eight patients. Hematologic, skin and soft tissue, and central nervous system tumors were the diagnosis in the remaining patients.
Men diagnosed with cancer rated their QOL on the VAS scale as being significantly worse than women (P = 0.02). This difference was not seen in the general OPD patients.
In the EQ5 domains, significantly greater problems were rated by the men in the domains of mobility, self-care and usual activities (P < 0.005) whereas pain and anxiety ratings were similar among the two sexes. People who had been referred to the pain services reported significantly worse QOL than those not (P = 0.01). When analyzed according to cancer stages, persons with stage two cancer reported maximum anxiety. Anxiety and depression were also significantly greater in cancer patients whose spouse were alive than not (P = 0.000).
There was no relation of education level or socioeconomic grade with the QOL as assessed by EQ5D domains or VAS scores.
DISCUSSION
Translation and validation of a tool across cultures is a challenge. The EQ5D has traditionally been validated and tested in the western population; although translations are available in other languages, few have attempted the validation of a translated non-European version.[18] Studies from India have recently started to use local translations of the English (Indian) version of the EQ5D, but to the best of our knowledge, we are the first to attempt validation of the translated tool in an Indian patient population.[1920]
The translation process, done in close collaboration with the EQ group was rigorous, taking more than 3 months. Similar to Kim et al., the group and the respondents found it mostly simple to translate and understand. Some words or phrases such as “anxiety,” “confined to bed,” “depression,” had to be discussed to reach a consensus equivalent having the right nuance as many words exist in Odia language varying in grade of severity for anxiety and depression.
The questionnaire was administered to the large percentage of uneducated patients by trained personnel in face to face interviews. The interviewers reported a difficulty in comprehension of the VAS scale by some people. The main reasons were two: A difficulty in grasping the mathematical concept of the measuring scale and a general apathy in trying to apply the concept. Application of a monetary scale (like 10 paise of a rupee to demonstrate 10 of 100) and gentle persuation overcame these hurdles in all patients.
The translated version fulfilled three of the four a - priori hypotheses for validation - the problems reported on the EQ5D descriptive domains increased significantly with age and Odia version could discriminate well between patients coming to the general OPD and patients with cancer, thereby demonstrating a good construct (convergent and discriminant) validity. In our population, unlike that seen by Cleemput et al., female patients did not report greater anxiety or depression than men-this may be a reflection of cultural difference, as Indian women are presumably exposed to greater suppression and hardships than their western counterparts.[21] The anxiety/depression domain of the EQ5D showed a strong correlation with all the WHO-5 domains-demonstrating the concurrent validity of the translated instrument; the correlation with other EQ5D domains was poor, as seen in earlier studies.[14] The internal consistency and test-retest reliability were also high for the translated version in our patient population.
The patients with cancer being admitted to the Regional Cancer Centre in the Eastern part of the country appear to have a poorer self-reported QOL – mean EQ5D index 0.37 ± 0.4 compared to data from Western countries (index means 0.33 ± 0.4–0.93 ± 0.12) cross patients with various types and stages of cancer.[22]
The reasons for a poor health status of cancer patients in India are multifactorial and our study was not designed to assess them.[23] However, the significantly worse QOL in patients referred to pain services might indicate a delay in referral to and dearth of palliative care and pain services in Odisha. As seen previously, patients with a lower stage of cancer (stage two) had significantly higher anxiety and depression.[24] This group lies midway between a curable pathology and acceptance of a poor prognosis-improved counseling and a multipronged approach with psychiatric assessment may be warranted in them. Contrary to Western studies, our female patients showed no difference in anxiety scores and reported problems in mobility were high among male patients. Anxiety and depression were significantly higher among cancer patients who had a living spouse, possibly indicating concerns in social support to the surviving spouse with worsening condition of the patient, again a possible cultural effect. We did not find any association with the level of education, socioeconomic status and QOL.
CONCLUSION
Odia version of the EQ5D has good reliability and validity for the measurement of health status in oncology and OPD patients and can be used in similar clinical scenarios. The QOL of cancer patients in our cohort appears low, and causes and interventions for the same will need to be further investigated. Our results being in line with existing evidence may allow the use of Odia EQ5D in the evaluation of economic and outcome measures in Eastern India.
The EQ5D being a simple generic tool can be administered even in the outpatient practice, validation of this translated version in other disease states will allow more widespread use.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Validity and reliability of MOS short form health survey (SF-36) for use in India. Indian J Community Med. 2013;38:22-6.
- [Google Scholar]
- Food and Drug Administration. Guidance to the Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Rockville: Food and Drug Administration; 2009.
- [Google Scholar]
- Use of quality-adjusted life years for the estimation of effectiveness of health care: A systematic literature review. Int J Technol Assess Health Care. 2006;22:235-41.
- [Google Scholar]
- Validation of the EQ-5D quality of life instrument in patients after myocardial infarction. Qual Life Res. 2005;14:95-105.
- [Google Scholar]
- Validity and responsiveness of the EQ-5D in assessing and valuing health status in patients with anxiety disorders. Health Qual Life Outcomes. 2010;8:47.
- [Google Scholar]
- Quality of life of patients with cancer in India: Challenges and hurdles in putting theory into practice. Psychooncology. 2004;13:429-33.
- [Google Scholar]
- Reliability and validity of the Malayalam hospital anxiety and depression scale (HADS) in cancer patients. Indian J Med Res. 2005;122:395-9.
- [Google Scholar]
- Validity of EuroQol – A generic health status instrument – In patients with rheumatoid arthritis. Economic and Health Outcomes Research Group. Br J Rheumatol. 1994;33:655-62.
- [Google Scholar]
- Reliability and validity of the EuroQol in patients with osteoarthritis of the knee. Rheumatology (Oxford). 1999;38:807-13.
- [Google Scholar]
- Measuring well-being rather than the absence of distress symptoms: A comparison of the SF-36 Mental Health subscale and the WHO-Five Well-Being Scale. Int J Methods Psychiatr Res. 2003;12:85-91.
- [Google Scholar]
- Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: A multi-country study. Qual Life Res. 2013;22:1717-27.
- [Google Scholar]
- Screening for depression in elderly Indian population. Indian J Psychiatry. 2010;52:150-3.
- [Google Scholar]
- Convergent and Discriminant Validity. 21 August, 2009. Explorable.com. Available from: http://www.explorable.com/convergent-validity
- [Google Scholar]
- Applied Statistics for the Behavioural Sciences. Vol xix. (2nd ed). Boston: Houghton Mifflin; 1988.
- [Google Scholar]
- Cross-cultural adaptation and validation of the Korean version of the EQ-5D in patients with rheumatic diseases. Qual Life Res. 2005;14:1401-6.
- [Google Scholar]
- Quality-of-Life among Elderly with Untreated Fracture of Neck of Femur: A Community Based Study from Southern India. J Family Med Prim Care. 2013;2:270-3.
- [Google Scholar]
- Assessment of effect of diabetes on health-related quality of life in patients with coronary artery disease using the EQ-5D Questionnaire. Value Health Reg Issues. 2014;3:67-72.
- [Google Scholar]
- The construct and concurrent validity of the EQ-5D in a renal transplant population. Value Health. 2004;7:499-509.
- [Google Scholar]
- Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics. 2007;25:365-84.
- [Google Scholar]
- Reasons for low quality of life in South Indian cancer patient population: A prospective observational study. Indian J Pharm Sci. 2014;76:2-9.
- [Google Scholar]
- Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro Oncol. 2008;10:171-81.
- [Google Scholar]






