Translate this page into:
Metronomic Chemotherapy in Anaplastic Thyroid Carcinoma: A Potentially Feasible Alternative to Therapeutic Nihilism
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Anaplastic thyroid carcinoma (ATC) is one of the most aggressive malignancies and prognostic outlook remains very dismal. Treatment most often is palliative in intent attempting to relieve the patients from local compressive symptoms in the neck. Radical surgery, radiotherapy (RT), and chemotherapy have not been tested in large prospective trials, and current evidence from retrospective series and small trials indicate only marginal survival benefits. Given the poor prognostic and therapeutic outlook, patients must be encouraged to be actively involved in the decision making process. We report the case of an elderly patient who had no response to palliative RT, and was treated with oral metronomic chemotherapy. The response to oral metronomic chemotherapy was dramatic, and the patient has enjoyed complete freedom from symptoms as well as radiologically exhibits a complete regression. Thus, we document the first ever use of a simple, cost-effective, and convenient oral metronomic chemotherapeutic regimen delivering a remarkable response in an elderly patient with ATC.
Keywords
Anaplastic carcinoma thyroid
Anaplastic thyroid cancer
Metronomic chemotherapy
Metronomic cyclophosphamide
Therapeutic nihilism
INTRODUCTION
Anaplastic thyroid carcinoma (ATC) representing just <5% of all thyroid malignancies accounts for about 50% of all deaths related to thyroid malignancies.[12] Since the disease is rare in absolute terms, there is a paucity of published literature and there is solid consensus upon a fixed standard of care. Most patients suffer pressure symptoms from local progression of the disease, and thus palliative procedures such as tracheostomy are often required.[3]
Surgical treatment is often limited to resection of the mass, and extensive neck dissection is avoided since undue morbidity must be avoided, especially in the lack of significant survival benefit. Radiotherapy (RT) too is largely palliative in intent and only marginal benefits can be expected after doses beyond 40 Gray (Gy).[456] Chemotherapy has traditionally been based on doxorubicin, and recently newer agents such as paclitaxel are being utilized recently. However, no large trials have been conducted, and the most appropriate chemotherapeutic agents are yet to be defined.[7] Since existing procedures have been shown only to be of marginal survival benefits, the individual patient deserves to be well-informed about the prognosis and treatment options. The patient must thus be actively involved in the decision-making process.
This report revolves around an elderly patient, who being well-informed about the treatment options and prognosis had approached us stating that she was against any form of invasive surgery, intravenous chemotherapy, or prolonged RT. We hence had mutually agreed for a short course palliative RT (20 Gy in five fractions). However, since the disease and the symptoms progressed despite RT and that the patient desired to have a treatment option which could be ‘had at the convenience of home, in the company of family’, the new approach of orally delivered metronomic chemotherapy was chosen.
The regimen (containing oral cyclophosphamide, methotrexate, and celecoxib) was designed with an emphasis on simplicity and low toxicity. The response to the same was very dramatic, with the patient relieved of dysphagia as early as after the first cycle. At the end of three cycles, there has been a clinical cessation of all symptoms and there has radiologically been a near complete response.
Thus, metronomic chemotherapy has not only provided relief from symptoms, but in this patient has induced a very good tumor response. In ATC where the dismal prognosis often encourages a sense of therapeutic nihilism on the part of the treating physician, the use of metronomic chemotherapy could prove to be a cost effective, convenient, and possibly effective treatment among patients who would otherwise be offered no treatment at all.
CASE REPORT
Prior to her presentation with us, the 67-year-old lady had visited a major hospital in the national capital for symptoms of a rapidly progressive mass in the neck with dysphagia. There she had been diagnosed as suffering from ATC [Figure 1], and recalls having being provided frank and ample information about prognosis and the available treatment options. The patient there had refused all forms of intravenous chemotherapy and surgery. She had also refused RT since she did not want to spend much time in a city away from home.
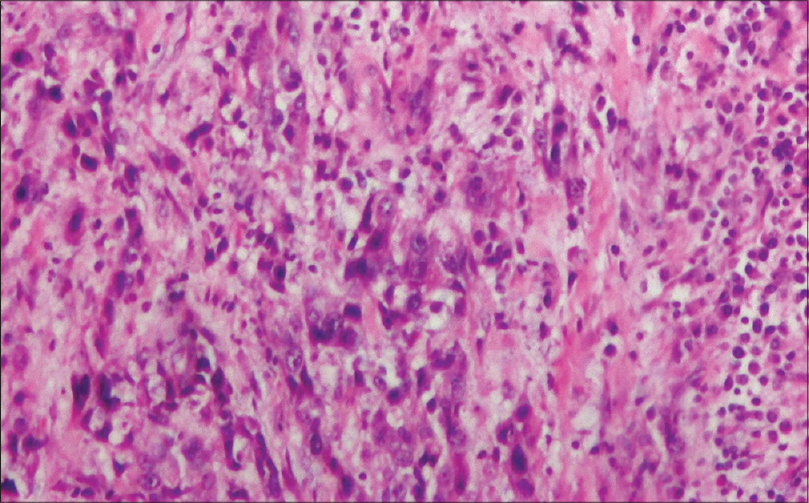
- Microscopic histopathological appearance of anaplastic cells present diffusely as well as in the form of cords in a desmoplastic stromal background. The tumor cells show bizarre nuclei, irregular nuclear border, pleomorphic reticular to hyperchromatic irregular chromatin
She presented to our department owing to close proximity to her residence. The already well-informed patient made aware to us that her main intention was to spend as much time as possible with her family at home, and that she was unwilling for intravenous chemotherapy, prolonged course of RT, or any form of surgery. Thus, a compromise was made to settle for a short course palliative RT of 20 Gy in five fractions. However, a month later, the patient reported progression of her symptoms, and there was clinical progression of the disease.
Though intravenous chemotherapy with either of doxorubicin or paclitaxel was again offered as an option, the patient refused the same and requested for a treatment which could be orally had at home. We thus discussed the new approach of orally delivered metronomic chemotherapy, and after a full discussion of the potential disadvantages and advantages of the new approach, the patient requested to be given the same. The metronomic chemotherapy delivered as a three-drug regimen, which was empirically designed as a pragmatic three-weekly cycle comprising oral cyclophosphamide (50 mg once a day on days 1–15); oral methotrexate (5 mg once a day on Mondays, Wednesdays, and Fridays); and oral celecoxib (200 mg once a day on Tuesdays, Thursdays, and Saturdays).
The patient experienced a dramatic response and after the first cycle, in that she was able to take oral feeds without the requirement of a nasogastric tube. After a further three cycles the patient is free of all previous symptoms. Computed tomography revealed a complete response [Figure 2]. The patient has tolerated six cycles of the regimen without any hematological toxicity.

- The presence of a large mass visibly causing obstruction of the esophagus (Panel A), which has completely regressed after four cycles of oral metronomic chemotherapy (Panel B)
Given the fact that the degree of response is highly unexpected in ATC, after the sixth cycle, the blocks were reviewed for the purpose of ruling out more chemosensitive tumors. Since only a scant volume of tissue was available from the preserved blocks, the same could only be processed for staining with three immunohistochemical stains—the leukocyte common antigen (LCA), neuron specific enolase (NSE), and CD99. This was done so as to rule out lymphoma and small round cell tumors, both of which are known for their chemosensitivity.
DISCUSSION
ATC holds the dreaded reputation of being an extremely aggressive and mostly incurable malignancy. The overall survival often ranges in a span of weeks to months, often despite multimodality treatment. Given the rarity of ATC in absolute terms, there is a paucity of clinical evidence with respect to the standard of care in the management of these patients. Once the diagnosis of ATC is established, the minimal stage that can be assigned is Stage-IV; with stage IVA implying ATC confined to the thyroid, stage IVB implying involvement of regional lymph nodes, and stage IVC implying distant metastases.[89]
While resection of disease often is done to provide symptomatic relief from pressure symptoms, it could also confer a slight survival advantage if combined with postoperative RT, especially with doses beyond 40 Gy. It is however not practical to force a patient into a prolonged course of RT, especially when most patients are left with few weeks of remaining life. The use of chemotherapy, mostly with doxorubicin in the past, and recently with newer agents such as taxanes and platinum compounds have also failed to provide lasting freedom from disease progression.[101112] Thus, the patients of ATC can only be expected to survive for short periods of time, and before treatment they must be provided with all information about treatment options and prognosis. The patients should hence be in charge of prioritizing their final days of life, as well as being involved fully in the decision making process.
Since the patient did not have any symptomatic benefit with RT (20 Gy in five fractions) and that the disease was seen to be progressive after a month, the patient was advised chemotherapy, which was refused. The patient demanded no further treatment, unless she could receive a form of treatment which could be had at home. We were thus faced with a situation where in the patient could either be offered some form of oral chemotherapy, or be offered nothing specific at all.
There have been many recent publications describing the use of metronomic chemotherapy, which essentially means the delivery of dense, uninterrupted administration of subtoxic doses of chemotherapy over protracted periods of time without prolonged drug-free intervals. It is delivered with the main aim of altering the tumor microenvironment by inhibiting tumor supporting vasculature, inducing tumor dormancy and possibly restoring immune surveillance. Metronomic chemotherapy has mostly been used in patients after prior treatment with various forms of conventional chemotherapy. The mechanism of action of metronomic chemotherapy is described as being different from conventional chemotherapy which is often delivered by the ‘maximum tolerable dose’ (MTD) approach.[1314151617]
Chemotherapy delivered conventionally by the MTD approach is mostly delivered in the form of temporally spaced cycles—meaning that a very high concentration of the drug is achieved in the initial days of the cycle, followed by a prolonged recovery phase of 10–15 days wherein there is negligible circulation of the chemotherapeutic agent. While the gaps between cycles are designed with an intention to allow adequate bone marrow recovery, it must also be noted that this can also allow for the recovery and proliferation of tumor cells.[1718]
Metronomic chemotherapy, while not being able to achieve high drug concentrations, however enables a continuous low dose exposure of the drug in the tumor microenvironment. This is likely to provide additional benefits not seen in MTD chemotherapy. While MTD chemotherapy focuses mainly on tumor cell cytotoxicity, metronomic chemotherapy is shown to act by various additional mechanisms, including antiangiogenic and immunological mechanisms. Since the tumor endothelium is much more sensitive to chemotherapy in comparison to tumor cells, especially at doses in the range of 1/10–1/50 times of the MTD, a continuous exposure to low doses of the cytotoxic agent could provide adequate antiangiogenic effect while being minimally toxic to the patient.[1920212223]
Metronomic chemotherapy acts against tumor vascular development, which could occur by direct cytotoxicity against the proliferative endothelial cells, upregulation of antiangiogenic factors, and the downregulation of angiogenic factors. Many conventional cytotoxic agents (e. g., cyclophosphamide) are known to have antiangiogenic effects, and it has been observed that their antiangiogenic effects are best noticed when the endothelium is exposed to the agent for prolonged break-free periods.[23] Metronomic chemotherapy has been shown to up regulate endogenous antiangiogenic factors such as thrombospondin (TSP-1) and angiostatin, while also down regulating endogenous angiogenic factors such as vascular endothelial growth factors (VEGF) and basic fibroblast growth factor (bFGF). In addition to being antiangiogenic, metronomic chemotherapy also inhibits vasculogenesis by depleting endothelial progenitor cells.[2425]
Metronomic chemotherapy can stimulate immune response by depleting T-reg (CD4+ CD25+) lymphocytes and also by inducing dendritic cell maturation. T-reg cells are regulatory T lymphocytes and increased T-reg numbers are associated with increased tumor progression and reduced treatment response. Metronomic chemotherapy has been shown to reduce T-reg lymphocytes, while not depleting other types of lymphocytes (MTD chemotherapy on the other hand causes a depletion of all forms of lymphocytes). The depletion of T-reg cells by metronomic chemotherapy allows the cytotoxic (CD8) and helper (CD4) T-lymphpocytes as well as natural killer cells to act unhindered in staging an antitumor immune response.[2425]
Metronomic chemotherapy is a very interesting option, given that it has been proven effective in many studies conducted in patients who were previously extensively treated with various lines of conventional chemotherapy. However, given that this is a new frontier in the art of chemotherapy, there exists a lack of consensus regarding the best agent, the optimal dose and the duration of treatment.
The reason for us designing a regimen containing cyclophosphamide, methotrexate, and celecoxib was based upon prior studies in other malignancies, as well as upon considerations such as local availability of the drug. Oral cyclophosphamide has been the most commonly utilized drug in studies on metronomic chemotherapy, and hence we included it in our regimen.[26] Though studies have utilized the drug given on a continuous daily basis, we provided it on days 1–15 every 20 day cycles due to concerns of potential marrow toxicity. We also utilized oral methotrexate 5 mg on Mondays, Wednesdays, and Fridays; while oral celecoxib 200 mg was used on Tuesdays, Thursdays, and Saturdays. It can be noticed that we utilized a rather cautious dose of methotrexate and celecoxib since we wanted to take no risk in causing patient any toxicity, while the option of escalating dose was reserved for further cycles.
However, since the patient did respond very well to the first cycle, with no toxicity for the doses used, the same regimen was continued. The response was dramatic, and admitably serendipitous.
However, we are unsure about the further course of action. While metronomic chemotherapy has been utilized for prolonged periods, there is no consensus regarding the duration of use. Though the prolonged use of cyclophosphamide has been known to be leukemogenic (such as rarely seen in patients taking cyclophosphamide for other indications such as autoimmune diseases), it must be however be stressed upon here that the intention was to address an existing issue and the distant risk of a second malignancy is not of major concern.
We to the best of our knowledge believe that this is the first ever case of ATC treated with, and responding well to metronomic chemotherapy. Despite good results in this individual patient, we cannot claim this as an alternative to existing treatment protocols in ATC. However, metronomic chemotherapy being associated with little or no toxicity can be offered to patients who could otherwise be subjected to therapeutic nihilism.
Before conclusive statements about metronomic chemotherapy can be made, more studies have to be published especially involving patients who are otherwise unfit, or unwilling for other forms of treatment. Metronomic chemotherapy could be an approach in the very old, the heavily pretreated, patients with poor performance status, patients with psychiatric contraindications to conventional therapy. In many advanced, disseminated, and heavily pretreated cases.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Outcome after intensity modulated radiotherapy for anaplastic thyroid carcinoma. BMC Cancer. 2014;14:235.
- [Google Scholar]
- Anaplastic thyroid cancer: A review of epidemiology, pathogenesis, and treatment. J Oncol 2011 542358
- [Google Scholar]
- Approach to the patient with anaplastic thyroid carcinoma. J Clin Endocrinol Metab. 2012;97:2566-72.
- [Google Scholar]
- Anaplastic thyroid cancer in British Columbia 1985-1999: A population-based study. Clin Oncol (R Coll Radiol). 2005;17:75-8.
- [Google Scholar]
- Concurrent doxorubicin and radiotherapy for anaplastic thyroid cancer: A critical re-evaluation including uniform pathologic review. Radiother Oncol. 2011;101:425-30.
- [Google Scholar]
- Surgery and radiotherapy improves survival in patients with anaplastic thyroid carcinoma: Analysis of the surveillance, epidemiology, and end results 1983-2002. Am J Clin Oncol. 2008;31:460-4.
- [Google Scholar]
- Anaplastic thyroid carcinoma: Dismal outcome despite current treatment approach. ANZ J Surg. 2004;74:559-62.
- [Google Scholar]
- The role of chemotherapy and latest emerging target therapies in anaplastic thyroid cancer. Oncol Targets Ther. 2013;9:1231-41.
- [Google Scholar]
- Thyroid. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual (7th ed). New York: Springer; 2010. p. :87-96.
- [Google Scholar]
- Treatment outcome of patients with anaplastic thyroid cancer: A single center experience. Yonsei Med J. 2012;53:352-7.
- [Google Scholar]
- A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer. 1985;56:2155-60.
- [Google Scholar]
- Reply to“Metronomic chemotherapy beyond misconceptions”-Haematologica 2013;98 (11):e145. Haematologica. 2013;98:e149-50.
- [Google Scholar]
- Antiangiogenic strategies on defense: On the possibility of blocking rebounds by the tumor vasculature after chemotherapy. Cancer Res. 2007;67:7055-8.
- [Google Scholar]
- Less is more, regularly: Metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105:1045-7.
- [Google Scholar]
- The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423-36.
- [Google Scholar]
- Metronomic chemotherapy: New rationale for new directions. Nat Rev Clin Oncol. 2010;7:455-65.
- [Google Scholar]
- Metronomic low-dose oral cyclophosphamide and methotrexate plus or minus thalidomide in metastatic breast cancer: Antitumor activity and biological effects. Ann Oncol. 2006;17:232-8.
- [Google Scholar]
- Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res. 2002;62:6938-43.
- [Google Scholar]
- Antiangiogenesis is produced by nontoxic doses of vinblastine. Blood. 1999;94:4143-55.
- [Google Scholar]
- Paclitaxel at ultra low concentrations inhibits angiogenesis without affecting cellular microtubule assembly. Anticancer Drugs. 2003;14:13-9.
- [Google Scholar]
- Metronomic chemotherapy: A new approach in cancer therapy. Postepy Hig Med Dosw (Online). 2008;62:364-71.
- [Google Scholar]
- Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878-86.
- [Google Scholar]
- Intermittent metronomic drug schedule is essential for activating antitumor innate immunity and tumor xenograft regression. Neoplasia. 2014;16:84-96.
- [Google Scholar]
- Metronomic chemotherapy: Possible clinical application in advanced hepatocellular carcinoma. Transl Oncol. 2013;6:511-9.
- [Google Scholar]
- Low-dose “metronomic chemotherapy” with oral cyclophosphamide and methotrexate in metastatic breast cancer: A case report of extraordinarily prolonged clinical benefit. Ecancer Med Sci. 2012;6:275.
- [Google Scholar]






