Translate this page into:
Audiometric Patterns in Ototoxicity After Radiotherapy and Chemotherapy in Patients of Head and Neck Cancers
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Inspite of various strategies adopted to protect the sensitive structures during organ preservation strategies, radiation damage can occur from the pharyngotympanic tube to the brain stem auditory pathway causing hearing loss. The purpose of this study is to evaluate the audiometric abnormalities and characterize them among the patients of head and neck cancers who have undergone radiotherapy (RT) and chemoradiation therapy (CT+RT).
Materials and Methods:
Sixty-six histopathologically proven head and neck cancer patients receiving RT and 34 patients receiving concomitant CT + RT underwent evaluation for audiometric abnormalities from 1st September 2010 to 31st August 2012.
Results:
Hearing losses were predominately of sensorineural type and mild. Patients who received concomitant CT+RT experienced greater sensorineural hearing loss compared with patients treated with RT alone. A paired sample t-test was conducted to compare the hearing losses before therapy and 6 and 12 months after therapy and was found to be significant (P < 0.05). It was found that hearing loss was persistent. Significant difference was found in the proportion of hearing loss after RT and RT+CT (P < 0.05) after 1 month. In addition, mixed hearing loss occurred due to damage to the middle ear contents and can be improved if intervened appropriately.
Keywords
Audiometric patterns
Chemotherapy
Radiotherapy
INTRODUCTION
Head and neck cancer is an emerging problem in India. About 70% of the affected patients present with clinically advanced disease, either at the primary site or in the cervical lymph nodes.[1] Locoregional control is critical in management of head and neck cancers as recovery is often difficult. Multimodal approach has been found to reduce the risk of local failure and improves survival. Strategies of organ preservation using RT and chemotherapy has been the main stay of treatment. Inspite of various strategies adopted to protect the sensitive structures such as brain stem, spinal cord, optic chiasma, cornea and the pituitary gland, no strategies have been adopted to protect the inner ear. Radiation damage can thus occur from the pharyngotympanic tube to the brain stem auditory pathway causing hearing loss. The purpose of the study is to evaluate the audiometric abnormalities among the patients of head and neck cancers who have undergone RT and chemoradiation therapy (CT+RT).
MATERIALS AND METHODS
One hundred patients were studied from 1st September 2010 to 31st August 2012. Criteria for inclusion:
-
Histopathologically confirmed cases of head and neck malignancies
-
Patients of head and neck cancers receiving RT alone and or concurrent CT+RT
-
Cases with Karnofsky's score ≥80%.
Criteria for exclusion:
-
Cases having bilateral severe sensorineural hearing loss, i.e. with bone conduction more than 60 dB
-
Patients with retrocochlear pathology
-
Karnofsky score <80%.
Of the 100 patients, 66 were treated by definitive RT, whereas 34 received concurrent chemoradiation therapy (RT+CT). All patients were evaluated before treatment with baseline audiogram. After completion of the full course of RT alone or with concurrent CT + RT, follow-up audiogram was performed after 1 month, 6 months and 1 year.
Audiological evaluation was done using Arphi-700 Mk IV diagnostic-research pure tone audiometer calibrated to ANSI-69 specifications. Hearing loss was classified according to World Health Organization (WHO) as follows: Normal: <15 dB, Slight: 16–25 dB, mild: 26–40 dB, Moderate: 41–55 dB, moderately severe: 56–70 dB, severe: 71–90 dB, Profound: >91 dB. Hearing loss of more than 15 dB either in the speech frequency, in the high frequency, or in both before and after therapy were considered significant.[234] To rule out retrocochlear pathology, Short Increment Sensitivity Index test was done. Treatment schedule: Site-specific treatment planning with a curative dose of 60–70 Gy units in 30–35 fractions with 1.8–2 Gy per day five fractions per week over 6–7 weeks. Concurrent cisplatinum-based weekly chemotherapy was administered in a dose of 30–35 mg/m2 given over 2–3 h of infusion.
Statistical analysis
Data entry was done in EPI INFO version 6.0 for analysis. Z-test was applied to calculate the difference in proportions of hearing loss due to RT and RT+CT. P <0.05 was considered significant.
RESULTS
Out of 100 patients, 66 (66%) received RT alone and 34 (34%) received concurrent CT+RT. Among the 100 cases of head and neck malignancies, oral lesions contributed the largest group (38%) followed by laryngeal (21%) and hypopharyngeal cancers (12%). Next in order were oropharyngeal, nasopharyngeal, nose, and para nasal sinus tumors. The smallest group was of occult primary with secondaries in neck with 2% of cases. The distribution of patients is shown below in Figure 1.
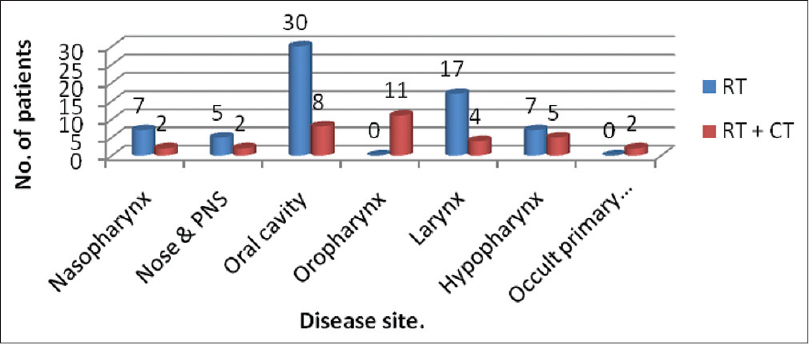
- Distribution of patients according to their sites
Type and classification of hearing loss after receiving RT alone
After 1 month, hearing losses of sensorineural type predominated, but occurrences of conductive hearing losses (≤6.06%) and mixed hearing losses (≤13.63%) were also seen in either ear. In the group of cases exposed to RT alone, mild hearing loss was observed in 57.57% of right ear and 51.51% of left ear and moderate hearing loss was observed in 16.66% in right ear and 9.09% in left ear. None of them had severe/profound hearing losses in either ear. After 6 months of therapy, sensorineural hearing loss predominated but occurrences of conductive hearing loss decreased to 1.51% in either ear whereas mixed hearing increased to 18.18% in right ear and 15.15% in left ear. The occurrences of mild and moderate hearing loss increased subsequently over time. In addition, severe hearing loss was seen in one left ear after 6 months and 1 year. Out of conductive hearing losses, none were significant clinically. Details of the observation are given in the Table 1.

Type and classification of hearing loss after receiving concurrent CT+RT
After 1 month, hearing losses of sensorineural type predominated with mixed hearing losses of ≤11.76% were seen in either ear. In this group, mild hearing loss was observed in 67.64% of right ear and 41.17% of left ear and 14.70% in right ear and 32.35% in left ear had moderate hearing loss. None of them had severe/profound hearing losses in either ear. After 6 months and 1 year of therapy, sensorineural hearing loss predominated and occurrences of mixed hearing increased to 14.70% in right ear and 17.64% in left ear after 6 months. The occurrences of mild and moderate hearing loss increased subsequently over time. In addition, severe hearing loss was seen in 1 left ear (2.94%) after 6 months and 1 year. Out of conductive hearing losses, none were significant clinically. Details of the observation are given in the Table 2.

A paired sample t-test was conducted to compare the hearing losses before therapy and 6 and 12 months after therapy and the losses were found to be significant (P < 0.05). It was found that hearing loss was persistent. The difference in proportions of hearing loss after RT and RT+CT (P < 0.05) after 1 month was found to be significant.
DISCUSSION
Hearing losses are predominately mild and of sensorineural type. Sensorineural hearing loss (SNHL) is caused by a lesion in the cochlea or retrocochlear component of the auditory system. The incidence increases in patients receiving concurrent CT+RT. In the present study, it was found that patients who received concomitant CT + RT experienced greater sensorineural hearing loss compared with patients treated with RT alone; these findings are consistent with the results previous studies reported by Low et al., Bhandare et al., and Schell et al.[567] Mixed hearing losses were mostly seen after RT to malignancy of nasopharynx. Depending on where the RT-induced lesion is located, the underlying cause for hearing loss differs. Damage to the components of middle ear, including eustachian tube and ossicular chain, may also occur. Eustachian tube dysfunction leading to otitis media with effusion, thickening of the tympanic membrane, and middle ear fibrosis can occur due to radiation. Tympanometric study of some patients revealed a B-type of curve, suggesting development of middle ear effusion, which also corroborated with the findings of mixed hearing loss. Schultz et al. in their study of hearing loss after RT in head and neck cancers also reported that hearing losses were mostly sensorineural (51.1%) and of mild degree with occurrences of conductive (≤0.7%) and mixed hearing loss (≤16.3%).[7] Hearing loss was mixed type in speech frequency and sensorineural in high frequencies. Anteunis et al. in their study reported that the initial air-bone gap was enlarged at 2 kHz and 4 kHz, indicative for changes of the middle ear induced by irradiation.[8] Mixed hearing loss was described as a chief finding after RT by different authors, after administering RT to malignancy nasopharynx and unilateral parotid tumor.[910] Mixed hearing loss is a transient sequelae of treatment. The duration of RT-induced secretory otitis media (3–6 months) should not be overlooked and it might be justified to insert a middle ear ventilation tube.[8]
Concern for the quality of life of patients undergoing cancer treatment is necessarily growing, and determination of hearing loss should be a part of investigations to enable better rehabilitation. Similar results were observed after concurrent treatment with cisplatin. However, the incidence of hearing loss increases.
CONCLUSIONS
-
The incidence and severity of hearing loss increase with time, especially at high frequencies
-
Patients who received concomitant CT+RT experienced greater sensorineural hearing loss compared with patients treated with RT alone
-
Hearing losses are predominately of sensorineural type and mild
-
Occurrence of mixed hearing loss is also present due to damage to the middle ear contents and can be improved if intervened appropriately
-
Concern for the quality of life of patients undergoing cancer treatment is necessarily growing, and determination of hearing loss should be a part of investigations to enable better rehabilitation.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Cancer control programme in india: Challenges for the new millennium. Health Admin. 2002;XVII:10-3.
- [Google Scholar]
- Sensorineural hearing loss in patients treated for nasopharyngeal carcinoma: A prospective study of the effect of radiation and cisplatin treatment. Int J Radiat Oncol Biol Phys. 1996;36:281-9.
- [Google Scholar]
- Prospective study of sensorineural hearing loss following radiotherapy for nasopharyngeal carcinoma. J Laryngol Otol. 2010;124:32-6.
- [Google Scholar]
- Sensorineural hearing loss after concurrent chemoradiotherapy in nasopharyngeal cancer patients. Radiat Oncol. 2011;6:19.
- [Google Scholar]
- Sensorineural hearing loss after radiotherapy and chemoradiotherapy: A single, blinded, randomized study. J Clin Oncol. 2006;24:1904-9.
- [Google Scholar]
- Ototoxicity after radiotherapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 2007;67:469-79.
- [Google Scholar]
- Hearing loss and complaint in patients with head and neck cancer treated with radiotherapy. Arch Otolaryngol Head Neck Surg. 2010;136:1065-9.
- [Google Scholar]
- A prospective longitudinal study on radiation-induced hearing loss. Am J Surg. 1994;168:408-11.
- [Google Scholar]
- Multiple late complications of irradiation treatment of nasopharyngeal carcinoma. Ear Nose Throat J. 1995;74:286-8.
- [Google Scholar]






