Translate this page into:
Evidence-based Clinical Practice Guidelines for Interventional Pain Management in Cancer Pain
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Intractable cancer pain not amenable to standard oral or parenteral analgesics is a horrifying truth in 10–15% of patients. Interventional pain management techniques are an indispensable arsenal in pain physician's armamentarium for severe, intractable pain and can be broadly classified into neuroablative and neuromodulation techniques. An array of neurolytic techniques (chemical, thermal, or surgical) can be employed for ablation of individual nerve fibers, plexuses, or intrathecalneurolysis in patients with resistant pain and short life-expectancy. Neuraxial administration of drugs and spinal cord stimulation to modulate or alter the pain perception constitutes the most frequently employed neuromodulation techniques. Lately, there is a rising call for early introduction of interventional techniques in carefully selected patients simultaneously or even before starting strong opioids. After decades of empirical use, it is the need of the hour to head towards professionalism and standardization in order to secure credibility of specialization and those practicing it. Even though the interventional management has found a definite place in cancer pain, there is a dearth of evidence-based practice guidelines for interventional therapies in cancer pain. This may be because of paucity of good quality randomized controlled trials (RCTs) evaluating their safety and efficacy in cancer pain. Laying standardized guidelines based on existing and emerging evidence will act as a foundation step towards strengthening, credentialing, and dissemination of the specialty of interventional cancer pain management. This will also ensure an improved decision-making and quality of life (QoL) of the suffering patients.
Keywords
Cancer pain
Celiac plexus block
Epidural opioids
Evidence-based
Guidelines
Interventions
Intrathecal drug delivery system
Lumbar sympathectomy
Neurolytic
Radiofrequency
Superior hypogastric
Vertebroplasty
INTRODUCTION
Pain is the symptom most prevalent and feared in oncology practice and has its direct implications upon patient's quality of life (QoL); perception of the effect of therapy, disease status, quality of services and even survival.[123] Intractable cancer pain resistant to World Health Organization (WHO) analgesic ladder afflicts 10–15% of cancer pain patients.[4] It is for this unremittable pain that the interventional therapies were introduced as the fourth analgesic step of the modified WHO analgesic ladder.[5] Etiologically, cancer pain is multifactorial and is characterized by diverse pathophysiological mechanisms consisting of nociceptive, neuropathic, and mixed mechanisms.[67] It is the neuropathic and bony metastasis pain which is resistant to conventional analgesics.[6] Interventional therapies have a specific role in management of cancer pain and constitutes of a plethora of techniques that includes minimally invasive neuroablative and neuromodulation interventions.[689] They are indicated when pain is resistant to or when intolerable adverse effects preclude the use of traditional pharmacotherapeutics. Rather than considering it as a standalone therapeutic measure, it should be considered as an indispensable component of multimodal pain management strategy.[8910111213] Practice guidelines review the existing literature and evidence to make them accessible and useful for clinicians, patients, and researchers alike.[11] They are of greater practical value when they are diagnosis specific and acts as a medico-legal shield when stringently adhered to in case of an unfortunate outcome.[1415]
GENERAL PREREQUISITES APPLICABLE TO ALL THE INTERVENTIONS
-
Patient must have received an optimal trial of analgesics as per WHO analgesic ladder and found to be recalcitrant or developed intolerable side-effects limiting their use or dose[6]
-
A detailed history and physical examination tailored towards etiology, quality, and putative anatomical transmission pathways involved
-
Accurate documentation of pain location, frequency, intensity, and its effect on QoL must precede interventions
-
Presence and degree of any neurological deficits, co-morbidities, drug allergies as well as any contraindications to interventions should be sought and well-documented at this stage[6]
-
Site-specific inspection at the intended puncture site to rule out any local infection or bed sores is a must and an absolute contraindication if found so. Patient's ability to lie in prone position for the duration of block should also be assessed[67]
-
Investigations including imaging and laboratory should be ordered and reviewed. Imaging may help with structural basis of pain (tumor compression), anatomical deviations (due to tumor), and sometimes in selecting the technique, approach, and needle trajectory for a safe and effective outcome. Recent coagulation profile, complete hemogram, random blood sugar, and other case-specific investigations must be ensured to be in normal/safe range before contemplating any invasive procedure
-
Written, informed consent preferably in patient's own language explaining the goals of the procedure, what to expect, financial implications, probable side-effects, and complication is a prerequisite.[6] Patients should be asked about any queries if they have which should be answered and documented. Consultation regarding preferred site of catheter exit and implantable pump should also be sought and respected
-
A diagnostic/prognostic block with local anesthetic to explore effectiveness, associated sensory and motor deficits should be contemplated before any therapeutic/neurolytic procedure[6]
-
Experience and familiarity of the interventionist with the said procedure, following strict aseptic techniques, use of image guidance, meticulous technique, pre procedure antibiotic cover, and checking of all the instruments to be used are the ones not to forget.
GENERAL CONTRAINDICATIONS TO ALL INTERVENTIONS
Absolute
-
Patient refusal
-
Local or systemic infection
-
Uncorrected coagulopathy (INR > 1.5, platelet count <50,000)
-
Lack of technical expertise
-
Uncertainty regarding the diagnosis
-
Uncooperative patient
-
Patients with opioid addiction or drug seeking behavior
-
Allergy to the drugs to be used.
Relative
-
Antiblastic chemotherapy and neutropenia
-
Neurological deficits must be documented prior to procedure.
INTERVENTIONAL PAIN MANAGEMENT GUIDELINES ACCORDING TO CLINICAL DIAGNOSIS AND PAIN SYNDROMES
Interventions for head and neck cancer pain
Pain is a common symptom in HNC with prevalence as high as 85% at diagnosis.[16] Neuropathic pain has been reported in 30% of HNC patients with up to 93% of patients with pain having mixed nociceptive and neuropathic characteristics.[1718] The etiology of HNC pain may be tumor itself, iatrogenic or incidental pain due to co-existing conditions.[19] The pain location may vary from dysphagia to pain of varying severity in head, face, mouth, ears, cervical, and shoulder region.[2021] HNC pain management is particularly challenging considering the rich neural innervation and regional functions of speech, deglutition, and oral intake acting as aggravating factors.[22] The oral mucosa is accusatively sensitive resulting in chemotherapy or radiotherapy induced mucositis and pain.[22] Up to 10–20% of HNC patients may have inadequate pain relief or unacceptable side-effects with pharmacological management.[23] It is for this subgroup that interventional techniques comprising nerve blocks, neuroablation, or intraspinal drug infusions are recommended.[24] The nerve blocks which have been employed successfully for HNC pain are trigeminal, glossopharyngeal, occipital, vagal, sphenopalatine ganglion, and cervical plexus.[24] A 3-step process of diagnostic, prognostic, and therapeutic blocks ensures proper patient selection and allows patient to experience block-associated numbness and other side-effects prior to neurolysis.[25] Occipital headaches due to skull base invasion can be managed symptomatically with occipital nerve blocks.[23] Percutaneous radiofrequencyrhizolysis of trigeminal nerve and its branches, glossopharyngeal nerve, and sphenopalatine ganglion are safe, effective, and can be employed to tackle intractable HNC pain. Maxillary and mandibular nerve blocks are effective for cancer pain in their respective anatomical territories. Pulsed radifrequency (RF) is recommended for maxillary and mandibular nerves as they are somatic nerves with large A delta fibers and thermo coagulation is not recommended.[26] The needle is inserted through the mandibular notch to contact the lateral pterygoid plate and then redirected either anterio-superiorly or posterior-inferiorly for maxillary and mandibular nerve block, respectively. A stimulating current of 50 Hz at 0.5 V is delivered through a 5-cm needle with 5 mm active tip to confirm paresthesias in the distribution of the nerve. After confirmation pulsed RF is performed at 42°C and 2–3 cycles of 120 seconds each.[26] Glossopharyngeal neuroablation either surgically or alcohol neurolysis has been attempted in the past for treatment of pain in base of tongue, pharynx, and tonsils.[26] As the glossopharyngeal nerve is a mixed nerve, therefore pulsed RF and not neuroablation is recommended. A needle is inserted either blind or fluoroscopically guided at the midpoint of the line joining the angle of the mandible and the mastoid process to contact styloid process at a depth of not more than 3 cm.[26] After aspirating for blood or cerebrospinal fluid (CSF), a sensory stimulating current of 50 Hz at 0.5 V is delivered to reproduce a concordant pain. Motor stimulation is done with 2 Hz at 0.5–2 V which should not cause stylopharyngeus contraction.[26] Lesioningis done at 42°C for 3 cycles of 120 seconds each. This should be used in conjunction with medical management. The glossopharyngeal nerve pulsed RF is particularly efficacious for breakthrough pain and helps in reducing or at least stabilizing the dose of opioid medications.
Interventions for intractable thoracic/chest wall cancer pain
Pain is the most common symptom of malignant chest wall tumors and usually indicates metastatic bony invasion.[27] Chest wall tumor invasion is often incurable with treatment focus being palliative care and pain control. Intractable pain or intolerable side-effects of WHO analgesic ladder frequently warrants interventions such as intercostal block, neurolysis, pulsed RF, or intrathecal pump implantation.[28] Intercostal block (ICB) is recommended under radiologic control with the needle inserted proximal to the angle of the rib at its lower border. After encountering, bone needle is advanced few millimeter deeper and the intercostal groove is confirmed with 0.5 ml of contrast. Overlapping intercostal nerve innervation necessitates the use of diagnostic blocks at three consecutive levels to identify the involved nerve. ICB with local anaesthetic and steroid act as a diagnostic block prior to neurolysis and may lead to prolonged pain relief in some patients.[28] NeurolyticICB (6–10% Phenol) is frequently employed for intractable chest wall pain as motor blockade is not a major concern.[29] Almost all the patient experience immediate pain relief however of short duration and up to 30% of patients may experience neuritis or deaffrentiation pain.[2930] Pneumothorax, although rare, can occur necessitating meticulous technique and radiologic guidance. The quality of evidence according to scoring system published by Guyatt et al., is 0 (efficacy demonstrated in case reports, to be considered only study related).[731]
Interventional techniques for upper abdominal cancer pain
The pancreatic cancer often being diagnosed in advanced stage is usually associated with 1 year and 5 year overall survival rates of 26% and 6%, respectively.[3233] Pancreatic ductal adenocarcinoma has a neural invasion rate of 80–100%.[3435] Severe abdominal pain is the major symptom in 70–80% of these patients and is often difficult to treat.[3637] Cancer cell infiltration of neural sheaths, tumor pressure upon nerves, altered expression of signaling molecules, neovascularization, and inflammation are some of the putative etiologies of pain.[323438] Pain is usually situated in the upper abdomen and sometimes back. It is diffuse, poorly localized, deep-seated, cramping, or colicky in nature, aggravates on lying down, and relieves on bending forward.[7] The pharmacological management is frequently associated with incomplete, inadequate pain relief as well as drug-related adverse effects like nausea, vomiting, constipation, dry mouth, and drowsiness.[32] Moreover, visceral pain mechanisms are dynamic and changes with disease progression necessitating neurolytic blocks as adjuvants to reduce opioid consumption.[39] Pancreatic cancer pain is mediated by splanchnic nerves via celiac plexus to brain and is amenable to treatment by neurolysis of two anatomically distinct structures: Celiac plexus and splanchnic nerves.[3240] The splanchnic nerves are preganglionic sympathetic afferent fibers, retro-crural in location at the level of twelfth thoracic vertebra and consist of greater (T5-T10), lesser (T10-T11), and least (T12) splanchnic nerves.[1041] These splanchnic nerves coalesce to form celiac plexus anterior to aorta surrounding the origin of celiac trunk. The celiac plexus consisting of 1–5 ganglia and is antero-crural in location at the level of T12-L2 vertebral bodies.[1040] It consists of pre and postganglionic, parasympathetic, and visceral afferent sensory fibers.[15] The celiac plexus carries nociceptive signals from upper abdominal viscera including the pancreas, gallbladder, distal portion of the stomach; small intestine, and large intestine up to the transverse colon.[10]
Neurolytic celiac plexus block
NCPB is the most common cancer pain intervention performed and is highly effective for upper abdominal visceral pain.[1042] Radiating lower abdominal or back pain due to upper abdominal malignancy is not a contraindication to NCPB.[7] The effectiveness of NCPB in pancreatic cancer has been demonstrated in numerous RCTs.[353743] A recent meta-analysis showed the visual analogue scale (VAS) scores and analgesics use to be significantly lower at 2, 4, and 8 weeks in CPB group compared with medical management.[32] A Cochrane review of RCTs found statistically significant difference in the mean VAS score and opioid consumption at 4 weeks in favor of CPB compared with control group in patients with unresectable pancreatic cancer pain.[44] Literature suggests that visceral and somatic pain responds more favorably to neurolytic blocks as compared to neuropathic pain.[8]
Timing of the block
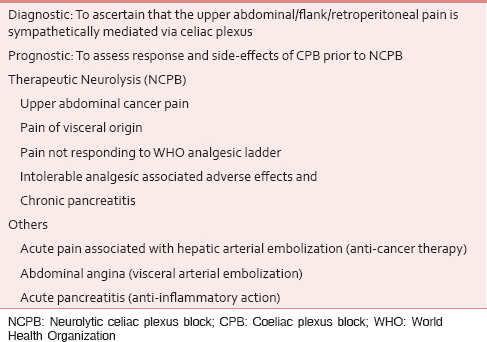
CPB should be considered early in the course of the illness where celiac axis is free and there is no contraindication as such an approach is associated with best pain relief, increased life expectancy, and last but not the least risks of neurolysis increases with disease progression.[245] Lillemoe et al., demonstrated that Ca pancreas patients who received CPB prior to severe pain had prevented or delayed development of pain, strengthening the evidence for role of CPB early in the course of disease.[2] Evidence-based indications [Table 1] and contraindications [Table 2] of CPB or splanchnic nerve block are as follows.

Technical considerations
NCPB
Since the introduction of percutaneous CPB technique by Kappis in 1914,[49] various approaches [Table 3] to access celiac plexus have been described. Literature does not show any statistically significant difference among them with respect to morbidity or results, although splanchnic nerve block with alcohol at T11 level appears to be more efficacious in terms of pain relief and QoL than the transaortic approach.[1055] The CPB can be performed with patient in prone or supine position via an anterior or posterior approach.[6] Image guidance inclusive of Fluoroscopy, ultrasonography (USG), or computerized tomography (CT) help guide accurate needle placement and reduce complication during percutaneous CPB.[615] CT guidance is expensive and continuous guidance throughout the procedure is not feasible.[15] The neurolytic agents most frequently applied for NCPB are alcohol (50–100%) and phenol (5–10%). Because of the high affinity of phenol for vascular structures, alcohol is the agent of choice for NCPB.[1556] As injection of alcohol is painful, it should be preceded by local anesthetic (LA) injection and the needles should be flushed with LA or saline after injection to prevent tracking of neurolytic agent.[7] The effect of neurolytic blocks is temporary persisting approximately for 3–6 months owing to axonal regeneration.[857]

Splanchnic nerve block
Although CPB and SNB have been used interchangeably, they are anatomically different. SNB involves blocking the splanchnics which are branches of thoracic sympathetic and constitutes nerve supply to the celiac plexus. This procedure is performed percutaneously with patient in prone position with the needle insertion in a tunnel view at the concave middle portion of the T11 vertebral body.[754] The needle should be in contact with T11 body, within its contour in AP fluoroscopic view and just at its anterior border in the lateral fluoroscopic view. A novel technique of CT-guided transdiscal approach of splanchnic nerve block has been described in patients with anatomic abnormalities/organomegaly to reduce the complications of paraplegia, pneumothorax, liver, or renal puncture.[58]
Adverse effects of NCPB
The main adverse effects are transient diarrhea (10–25%), orthostatic hypotension (20–42%), and local pain.[732] Other complications described in case reports include paresis, paresthesias (1%), hematuria, pneumothorax, shoulder pain (1%), hemorrhagic gastritis duodenitis, and death (3.1%).[7325960] However, most of the adverse effects are rare or transient and safety of CPB has been demonstrated in numerous observational studies, RCTs, and meta-analyses.
Failure rates: A plethora of literature exists to support the favorable outcome with NCPB with success rate of approximately 85% (70–100%) in pancreatic carcinoma patients.[155159] The block effect is temporary with the probability of pain recurrence increasing with increased survival necessitating a repeat block.[15]
Recommendations
CPB appears to be safe and effective for pain relief in patients with pancreatic cancer, with significant advantage over standard analgesic therapy [II B].[39] The quality of evidence according to scoring system published by Guyatt et al., is 2 A+ (highest level of evidence, positive recommendation).[714] The level of evidence for endoscopic ultrasound (EUS) celiac plexus neurolysis is B (single RCT/nonrandomized studies) with IIA recommendation (useful).[41] The level of evidence and recommendation for neurolytic SNB is 2 B+ (RCTs with methodological weakness, positive recommendation). The interventional treatment may be considered as soon as opioids are started.[7]
Interventional techniques for cancer-associated pelvic and perineal visceral pain
Pelvic cancers may result in excruciating pain unresponsive or associated with intolerable side-effects with oral opioids.[61] Oncologic cancer pain can be visceral (tumor involvement), somatic (impairment of pelvic musculature), or neuropathic (tumor infiltration or pressure upon neural structures).[62] About 75% of patients will present with pain any time during disease, 50% and 30% will have moderate-severe and very severe pain, respectively.[63] As visceral pain in lower abdomen is a major component in these patients Neurolytic Superior Hypogastric plexus Block (NSHB) should be employed more often.[7]
Neurolytic superior hypogastric plexus block
The afferent fibers innervating the pelvic viscera (the bladder, uterus, vagina, prostate, testes, urethra, descending colon, and rectum) travel with sympathetic nerves, ganglia, superior hypogastric plexus (SHP), and therefore are amenable to percutaneous NSHB.[76465] The SHP is a retroperitoneal structure located bilaterally from L3 to upper third of S1, close to sacral promontory and common iliac veins bifurcation.[764] Schmidt et al., reviewed the available literature and found NSHB to be safe and effective.[64] Mishra et al., in a recently conducted RCT reported NSHB to be superior to oral morphine with respect to pain score reduction, improvement in functional capacity, and global satisfaction score.[66]
The indications for NSHB are: Pelvic visceral pain, pelvic cancer pain, chronic non-cancer pelvic pain (endometriosis), and refractory penile pain.[64] The posterior approach is commonest but an anterior approach has also been described.[66] Plancarte et al., first described the classical technique in which needles were inserted bilaterally at the level of L5 and S1 vertebrae non-fluoroscopically in prone position, with 70–90% of patients achieving significant pain relief.[67] de Leon-Casasola et al., employed fluoroscopy during classic approach, with 69% of patients achieving satisfactory pain relief.[61] Several techniques to overcome the anatomic barriers (L5 transverse process and high iliac crest) encountered during classic approach have been described including transvascular,[68] transdiscal,[69] USG, and CT-guided anterior and posterior approach.[6566] The complications include injury to common iliac vessels, pelvic viscera, L5 nerve root, and discitis which are rare and can be avoided with proper attention to technique, preoperative antibiotics, and image guidance.
Recommendations
The quality of evidence according to scoring system published by Guyatt et al., is 2 C+ (benefits closely balanced with risks; considered, preferably study-related).[714]
Neurolytic inferior hypogastric plexus block
The inferior hypogastric plexuses are located in the presacral tissues lying ventral to S2-4 vertebrae medial to the sacral foramen.[70] NIHB is effective in terms of mean reduction in pain score and opioid consumption for pelvi-perineal pain conditions arising from lower pelvic organs and genitalia as they are frequently spared by NSHB.[7071] Schultzfirst described the fluoroscope-guided transsacral approach to inferior hypogastric plexus block to treat chronic lower pelvic pain.[71] The risk of transient paresthesias and rectal injury can be reduced by careful attention to technique and use of fluoroscopic guidance as described by Schultz et al.[71] Large prospective RCTs evaluating the safety and efficacy of this relatively new technique are warranted.
Ganglion impar block and neurolysis
Ganglion Impar or Walther is an unpaired structure located at the termination of bilateral lumbosacral sympathetic chains and supplies nociceptive and sympathetic fibers to the perineum, distal rectum, perianal region, distal urethra, vulva/scrotum, and the distal third of the vagina.[727374] It is a retroperitoneal structure located at or slightly below (up to 2 cm) the sacrococcygeal junction.[75] It is the sustained visceral sympathetically mediated perineal pain which is amenable for ganglion impar block.[76] Patients often complains of burning sensation or urinary and rectal urgency with ganglion impar associated dysfunction.[8] Other indications include radiation induced proctitis (rectal pain),[77] coccygodynia, perianal sweating, and tenesmoid pain.[78] Plancarte first described the technique for fluoroscopic-guided Ganglion impar block by introducing a bent needle through anococcygeal membrane with a finger inserted in the rectum to guide the needle and prevent rectal injury.[79] Other approaches described in the literature include transsacrococcygeal and transdiscal approach.[7280] However, fluoroscopic confirmation of retroperitoneal needle tip location and appropriate dye spread (Comma sign produced by 1–2 ml of radiographic contrast medium) is a must before injection of neurolytic agents (6% phenol) when USG is used as the primary imaging modality.[727981] The closer the needles tip to the rectum, the lower the volume of neurolytic agent (1–8 ml) that should be injected.[8] The complications include rectal injury, injury to nerves, and neuritis. The use of radiofrequency ablation of gastrointestinal (GI) which has been described for non-malignant perineal pain in pain of cancer origin may help in reducing the complications associated with chemical neurolysis.[73]
NEUROLYSIS OF LOWER SACRAL ROOTS (NEUROLYTIC SADDLE BLOCK)
Intrathecal phenolization may be considered for somatic perineal pain due to pelvic malignancies.[7] It is usually employed as a last resort for intractable cancer pain in terminal patients with pre-existing urinary catheter, artificial anus, or urinary and fecal incontinence.[7] It is contraindicated in patients with life expectancy ≥6 months or coagulation abnormalities. The technique involves slow intrathecal injection of 6% phenol in glycerin through a 22 g spinal needle introduced through L5-S1 interspace with the patient seated and leaning backward at an angle of 45° to maximize the flow towards dorsal or sensory rootlets.[78] The patient should remain seated in the above position for 6 hours. The quality of evidence according to scoring system published by Guyatt et al., is 0 (efficacy demonstrated in case reports, to be considered only study-related).[7]
INTRASPINAL INTERVENTIONAL PAIN MANAGEMENT TECHNIQUES
Intraspinal techniques rely upon medication delivery in close proximity to afferent nociceptive fibres and ascending tracts.[78] The delivered medication acts upon spinal receptors such as N Methyl D Aspartate (NMDA), sodium, calcium, and opioid channels involved in pain modulation.[8] The intraspinal techniques can be classified further into epidural and intrathecal depending upon the anatomic site of medication delivery.[8] For both administration routes, three drug delivery systems exist: Externalized, partially internalized, and fully internalized implantable systems. The life expectancy should ideally determine which system is to be used with implantable systems to be preferred over external pumps for life expectancy of ≥3 months.[82] The fully implanted system is associated with reduced infection and lower maintenance but is associated with higher upfront cost and technical expertise.[883] Epidural route is preferred for focal analgesia and shorter life expectancy and intrathecal route for wider area of analgesia and life expectancy of more than few weeks.[784] The evidence-based indications and contraindications for neuraxial analgesic techniques are [Tables 4 and 5, respectively].


Epidural infusion of drugs
Epidural analgesia can provide satisfactory pain relief in intractable cancer pain with efficacy varying from 76–100%.[88586] Catheters can be inserted, tunneled subcutaneously, attached to infusion systems and can be maintained for long periods.[8] However, clinical data supports the use of intrathecal catheters for more than 3 weeks.[87] Epidural infusion requires greater dosages, larger volumes, and more frequent refills as compared to externalized intrathecal catheters resulting in higher costs and infection rates.[8] The epidural infusion systems are associated with high complication rates (43–69%) which includes catheter dislocation/obstruction, infection, nausea, vomiting, drowsiness, constipation, and dural fibrosis.[858688]
Intrathecal infusion of drugs
Intrathecal infusion of drugs can be accomplished either by externalized intrathecal catheters or implantable drug delivery systems (IDDS).[8] The safety and efficacy of externalized intrathecal catheters in advanced cancer pain has been demonstrated even for periods extending up to 1.5 years.[8990] Intrathecal morphine is more effective, has less adverse effects, requires more compact and portable infusion system with longer period to refill as compared to epidural infusion systems.[89192] Home-based intrathecal infusion is cheaper, associated with improved analgesia and QoL.[92]
Intrathecal drug delivery system
IDDS for treatment of chronic pain were introduced in 1980 to deliver a fixed continuous rate of opioid infusion intrathecally.[8] Any change in dosage required refilling the pump with different concentration of medications.[9394] Externally programmable battery-operated implantable pumps were introduced in 1991.[93] This advancement facilitated easy non-invasive dose alterations in concert with dynamic nature of cancer pain.[94] Early implantation of IDDS is associated with prolonged survival rates.[95] An IDDS consists of an intrathecal catheter tunneled subcutaneously across the flank and connected to a small electronic pump with a battery life of up to 7 years implanted in the anterior abdominal wall which can be controlled by an external hand held control.[8] The use of IDDS is associated with lower costs in long term, improved mobility, QoL, ease of use, and prolonged survival.[96] Smith et al., reported IDDS with medical management to result in better analgesia; reduced drug-related adverse effects, and improved survival rates compared with medical management alone.[94] Ballantyne in a Cochrane review reported the clinical success rate of intrathecal morphine and conventional opioids for cancer pain to be 85% and 71%, respectively.[97] Potential complications include catheter related complications/fibrosis (5%), infection (2%); drug-related adverse effects, inflammatory mass around catheter tip, battery failure, and hardware malfunction.[9899] A trial of intraspinal analgesia to assess pain, function, mood, and adverse effects should always be contemplated before permanent implantation.[93100] A 50% decrease in pain along with favorable adverse effect profile is considered prognostic of sustained success with IDDS.[101]
Intrathecal medications
An array of intraspinal medications for both somatic/visceral pains (opioids, LA's) as well as neuropathic pain (opioids, LA, ziconotide, clonidine, baclofen) is used either alone or in different combinations.[8] The most frequently used medications include morphine (FDA approved), fentanyl, bupivacaine, ropivacaine, and clonidine.[7] Intrathecal clonidine can be added to opioids or local anaesthetics in doses of 150–600 μg/day as it enhances analgesia and reduces opioid related side-effects.[78102] Bupivacaine, like clonidine, is particularly useful for neuropathic or mixed cancer pain.[103] Ziconotide, an N type calcium channel blocker is FDA approved for intrathecal use and has been used effectively for treatment of chronic and cancer pain.[104] However, significant cognitive impairment and psychiatric changes associated with its usage warrants slow upward dose titration.[8]
Oral/SC/IV to epidural/intrathecal dose conversion: 300 mg/24 hours (oral morphine): 100 mg/24 hours (SC/IV morphine): 10 mg/24 hours (epidural morphine): 1 mg/24 hours (intrathecal morphine). Recommended insertion sites: The needle insertion site should be 10–15 cm away from intended catheter tip position. An intrathecal catheter should always be inserted at lumbar level below L1-L2.[7]
Recommendations
Intraspinal techniques for refractory cancer pain should be provided under supervision of a skilled team as a part of cancer pain management. However, their widespread use should be avoided [IIB].[39] The quality of evidence according to scoring system published by Guyatt et al., for intrathecal drug delivery is 2B+ and epidural drug delivery is 2C+ (considered, preferably study-related).[714]
Vertebroplasty and kyphoplasty
Bony metastases are common and 30–80% of bony metastasis involves vertebrae with most common primary sources being lung, breast, and prostate.[7105] These interventions are recommended for treatment of spinal pain due to vertebral collapse secondary to bony metastasis or osteoporosis without the involvement of spinal canal and its contents.[106107] Vertebroplasty involves stabilization of pathological fractures by injection of bone cement polymethylmetacrylate (PMMA), whereas Kyphoplasty involves percutaneous placement of intravertebral balloon, its inflation to restore vertebral height and reduce kyphotic angulation prior to injection of PMMA.[710] Kyploplasty as compared to vertebroplasty is more costly, technically more difficult, is associated with less pain relief and cement extravasation into spinal canal.[10106] The quality of evidence for treatment of painful pathological vertebral fractures according to scoring system by Guyatt et al., is 2B+ (considered, preferably study-related).[7]
Role of early interventions in cancer pain
With improving insights into mechanisms, neuroanatomical pain transmission pathways, increasing expertise in interventional analgesic therapies, and emerging evidence, there is a rising call for early implementation of interventional pain management in carefully selected group of patients.[1036108109] Evidence is building about the pervasive effects of long term opioid use such as progressive central sensitization, immune suppression, hypogonadism, cognitive dysfunction, and psycho-social implications.[110111] These may hinder the ability of cancer survivors to return to a normal life forcing some to suggest that the long practiced WHO ladder be turned “upside down” with early implementation of interventions.[112] Neurolytic blocks can provide prolonged pain relief, avoid distressing opioid-related side-effects and hence play a major role in palliation of intractable pain conditions.[113114115] This will be particularly advantageous in developing countries where inaccessibility and unavailability of opioids still prevails and hinders effective pain management.[108]
When to avoid interventions
A standardized patient evaluation, formulation of accurate clinical diagnosis is mandatory as interventions are target specific and proper patient selection increases the success ratio.[14116] Interventions should usually be reserved for well-defined and well-localized pain. Overzealous use of interventions in presence of widespread pain ormetastasis should be avoided as they are going to be futile and counterproductive. Nerve blocks in patients with multifocal pain may unmask pain at other sites; therefore, continuous use and monitoring of analgesia is crucial. The dynamic nature of the pain owing to the progressive disease and poor physical status not only poses tough challenges but also reduces the margin of error for palliative analgesic interventions. The interventionist performing the interventions should be properly trained, well-versed both theoretically as well practically with the indications, contraindications, approaches, preparation, and the troubleshooters of the procedure being performed. Rigorous training (Post-Doctoral Certificate Courses/Fellowships spanning over atleast 3 months to 1 year) in conjunction with cadaveric/mannequin hands on experience and clinical practice initially under expert supervision only ensures a competent interventional pain physician. Neither the poor prognosis of the cancer patients nor attending few day workshops makes one justifiable to perform these complex interventions ethically as well as medico-legally. The clinical skills of the less competent should be honed by observation, expert guidance, cadaveric/mannequin practice, and not by practicing upon cancer patients as guinea pigs. One should not forget that the ultimate goal of these interventions is to reduce and not increase the suffering. It is also the responsibility of the patients and their relatives to ascertain the qualifications and expertise of the interventional pain physicians in their localities from whatsoever resources available and opting for the best.
Limitations of interventional pain management techniques in palliative care practice
-
Multiple sites
-
Multiple types
-
Dynamic pain
-
Poor performance status.
CONCLUSION
Intractable cancer pain resistant to WHO analgesic ladder is a therapeutic nightmare for oncologists and pain physicians alike with significant effect on patient's QoL. Most of these cases can be managed effectively with various interventional pain techniques discussed in this article. These neurolytic or neuromodulation techniques should be employed in conjunction with medical management as a part of interdisciplinary treatment. Early institution of analgesic interventions helps not only in effective pain management, reduction in opioid doses, their side-effects but also have a survival benefit. Technical expertise, proper infrastructure, trained paramedical staff, and proper patient selection is of utmost importance for sustained safety and efficacy. Simple blocks such as trigger point injections can be performed in a hospice setting, whereas complex interventions must be contemplated in a hospital setting. One should adhere to the dictum of “Right intervention, in right person, by the right person, at the right time and place” in order to have sustained and effective results. Evidence-based medicine denies using intuition as the sole basis of clinical decision-making and requires critical review of available literature.[68] Further research in terms of properly conducted prospective blinded RCTs is warranted to lay down the indications and clinical scenarios in which these interventions are going to be most useful.
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- Retraction. Regarding: The fifth vital sign-what does it mean? Pain Pract. 2008;6:417-22. Pain Pract 2009;9:245
- [Google Scholar]
- Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann Surg. 1993;217:447-55.
- [Google Scholar]
- Anesthesiological interventions for the management of cancer pain. In: Schmidt RF, Willis WD, eds. Encyclopedic Reference of Pain. Heidelberg: Springer-Verlag; In Press
- [Google Scholar]
- Long-term patterns of morphine dosage and pain intensity among cancer patients. Hosp J. 1999;14:35-47.
- [Google Scholar]
- World Health Organization. WHO's pain relief ladder. Available from: http://www.who.int/cancer/palliative/painladder/en/
- [Google Scholar]
- The evolving role of interventional pain management in oncology. J Support Oncol. 2004;2:491-500. 503
- [Google Scholar]
- Interventional pain treatments for cancer pain. Ann N Y Acad Sci. 2008;1138:299-328.
- [Google Scholar]
- Interventional procedures for cancer pain management. Einstein (Sao Paulo). 2012;10:292-5.
- [Google Scholar]
- Interventional therapies for management of cancer pain. J Support Oncol. 2010;8:52-9.
- [Google Scholar]
- American Society of Interventional Pain Physicians. Interventional techniques: Evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7-111.
- [Google Scholar]
- The role of interventional therapies in cancer pain management. Ann Acad Med Singapore. 2009;38:989-97.
- [Google Scholar]
- Evidence-based interventional pain medicine according to clinical diagnoses. Pain Pract. 2011;11:423-9.
- [Google Scholar]
- Coeliac plexus blockade and neurolysis: An overview. Indian J Anaesth. 2006;50:169-77.
- [Google Scholar]
- Validation of world health organization guidelines for pain relief in head and neck cancer. A prospective study. Ann Otol Rhinol Laryngol. 1993;102:342-8.
- [Google Scholar]
- Identifying neuropathic pain in patients with head and neck cancer: Use of the leeds assessment of neuropathic symptoms and signs scale. J R Soc Med. 2003;96:379-83.
- [Google Scholar]
- Assessment of cancer pain: A prospective evaluation in 2266 cancer patients referred to a pain service. Pain. 1996;64:107-14.
- [Google Scholar]
- Neuropathic and nociceptive pain in head and neck cancer patients receiving radiation therapy. Head Neck Oncol. 2009;1:26.
- [Google Scholar]
- Pain management in patients with head and neck carcinoma. Otorhinolaryngol Clin. 2010;2:69-75.
- [Google Scholar]
- Pain control in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 1998;86:85-9.
- [Google Scholar]
- Head and neck nerve blocks for cancer pain management. Tech Reg Anesth Pain Manage. 1997;1:3-10.
- [Google Scholar]
- Review of chest wall tumors: A diagnostic, therapeutic, and reconstructive challenge. Semin Plast Surg. 2011;25:16-24.
- [Google Scholar]
- A retrospective review and treatment paradigm of interventional therapies for patients suffering from intractable thoracic chest wall pain in the oncologic population. Pain Med 2014
- [Google Scholar]
- Intercostal nerve blockade for cancer pain: Effectiveness and selection of patients. Hong Kong Med J. 2007;13:266-70.
- [Google Scholar]
- Nerve blocks in advanced cancer. Practitioner. 1982;226(539):541-4. Swarm RA, Karanikolas M, Cousins MJ. Anaesthetic techniques for pain control. In: Doyle DD, Hanks G, Cherny NI, Calman SK, editors. Oxford textbook of palliative medicine. 3rd ed. New York: Oxford University Press; 2005. p. 378-96
- [Google Scholar]
- Celiac plexus block for treatment of pain associated with pancreatic cancer: A meta-analysis. Pain Pract. 2014;14:43-51.
- [Google Scholar]
- American Cancer Society, Cancer Facts and Figures. 2012. Atlanta: American Cancer Society; Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document//acspc-031941.pdf
- [Google Scholar]
- Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695-707.
- [Google Scholar]
- Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: A randomized controlled trial. JAMA. 2004;291:1092-9.
- [Google Scholar]
- The effects of early or late neurolytic sympathetic plexus block on the management of abdominal or pelvic cancer pain. Pain. 2004;110:400-8.
- [Google Scholar]
- The effects of alcohol celiac plexus block, pain, and mood on longevity in patients with unresectable pancreatic cancer: A double-blind, randomized, placebo-controlled study. Pain Med. 2001;2:28-34.
- [Google Scholar]
- Pancreatic cancer pain and its correlation with changes in tumor vasculature, macrophage infiltration, neuronal innervation, body weight and disease progression. Pain. 2005;119:233-46.
- [Google Scholar]
- ESMO Guidelines Working Group. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23:vii139-54.
- [Google Scholar]
- Three posterior percutaneous celiac plexus block techniques. A prospective, randomized study in 61 patients with pancreatic cancer pain. Anesthesiology. 1992;76:534-40.
- [Google Scholar]
- EUS-guided celiac plexus neurolysis and celiac plexus block. Gastrointest Endosc. 2003;57:923-30.
- [Google Scholar]
- Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer pain. Pain. 1996;64:597-602.
- [Google Scholar]
- Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev 2011:CD007519.
- [Google Scholar]
- Coeliac plexus block for hepatic artery embolization: A comparison with intravenous morphine. Anesth Analg. 1989;69:398-9.
- [Google Scholar]
- One needle transcrural celiac plexus block. Single shot or continuous technique, or both. Reg Anesth. 1994;19:277-83.
- [Google Scholar]
- A new approach to the neurolytic block of the coeliac plexus: The transaortic technique. Pain. 1983;16:333-41.
- [Google Scholar]
- New technique for the neurolytic coeliac plexus block: The transintervertebral disc approach. Anesthesiology. 1996;85:212-7.
- [Google Scholar]
- Percutaneous anterior approach to the coeliac plexus using ultrasound. Br J Anaesth. 1989;62:637-40.
- [Google Scholar]
- Sympathetic and visceral nerve blocks. Clinical Procedures in Anesthesia and Intensive Care 1992:787.
- [Google Scholar]
- Efficacy of coeliac plexus and splanchnic nerve blockades in body and tail located pancreatic cancer pain. Eur J Pain. 2004;8:539-45.
- [Google Scholar]
- Neurolysis. Pharmacology and drug selection. In: Patt RB, ed. Cancer Pain. Philadelphia: JB Lippincott; 1993. p. :343-58.
- [Google Scholar]
- Intrathecal neurolytic blocks for the relief of cancer pain. Best Pract Res Clin Anaesthesiol. 2003;17:407-28.
- [Google Scholar]
- Transdiscal percutaneous approach of splanchnic nerves. Cir Cir. 2003;71:192-203. Article in Spanish
- [Google Scholar]
- Neurolytic celiac plexus block for treatment of cancer cancer pain: A meta-analysis. Anesth Analg. 1995;80:290-5.
- [Google Scholar]
- Paraplegia following intraoperative celiac plexus injection. J Gastrointest Surg. 1999;3:668-71.
- [Google Scholar]
- Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Pain. 1993;54:145-51.
- [Google Scholar]
- Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Reg Anesth. 1997;22:562-8.
- [Google Scholar]
- Is superior hypogastric plexus block effective for treatment of chronic pelvic pain? Rev Bras Anestesiol. 2005;55:669-79.
- [Google Scholar]
- Comparative study between computed tomography guided superior hypogastric plexus block and the classic posterior approach: A prospective randomized study. Saudi J Anesth. 2014;8:378-83.
- [Google Scholar]
- Efficacy of the anterior ultrasound-guided superior hypogastric plexus neurolysis in pelvic cancer pain in advanced gynecological cancer patients. Pain Med. 2013;14:837-42.
- [Google Scholar]
- Superior hypogastric plexus block for pelvic cancer pain. Anesthesiology. 1990;73:236-9.
- [Google Scholar]
- Superior hypogastric block: Transdiscal versus classic posterior approach in pelvic cancer pain. Clin J Pain. 2006;22:544-7.
- [Google Scholar]
- Chemical neurolysis of the inferior hypogastric plexus for the treatment of cancer-related pelvic and perineal pain. Pain Res Manag. 2013;18:249-52.
- [Google Scholar]
- Inferior hypogastric plexus blockade: A transsacral approach. Pain Physician. 2007;10:757-63.
- [Google Scholar]
- Blockade of the ganglion impar (Walther), using ultrasound and a loss of resistance technique. Prague Med Rep. 2012;113:53-7.
- [Google Scholar]
- Thermocoagulation of the ganglion impar or Ganglion of Walther: Description of a modified approach. Preliminary results in chronic, nononcological pain. Pain Pract. 2005;5:103-10.
- [Google Scholar]
- Interventional therapies for controlling pelvic pain: What is the evidence? Curr Pain Headache Rep. 2010;14:22-32.
- [Google Scholar]
- Clinical implications of topographic anatomy on the ganglion impar. Anesthesiology. 2004;101:249-50.
- [Google Scholar]
- Superior hypogastric plexus block and ganglion impar. Tech Reg Anesth Pain Manag. 2005;9:86-90.
- [Google Scholar]
- Successful treatment of radiation-induced proctitis pain by blockade of the ganglion impar in an elderly patient with prostate cancer: A case report. Pain Med. 2013;14:662-6.
- [Google Scholar]
- Ganglion impar block with botulinum toxin type A for chronic perinealpain-a case report. Korean J Pain. 2010;23:65-9.
- [Google Scholar]
- Presacral blockade of the ganglion of Walther (ganglion impar) Anesthesiology. 1990;73:A751.
- [Google Scholar]
- Modified approach to block the ganglion impar (ganglion of Walther) Reg Anesth. 1995;20:544-5.
- [Google Scholar]
- A modified needle-inside-needle technique for the ganglion impar block. Can J Anesth. 2004;51:915-7.
- [Google Scholar]
- Practical issues when using neuraxial infusion. Oncology (Williston Park). 1999;13:37-44.
- [Google Scholar]
- Epidural and subcutaneous morphine in the management of cancer pain: A double-blind cross-over study. In: Pain. Vol 67. 1996. p. :443-9.
- [Google Scholar]
- Epidural opiates and local anesthetics for the management of cancer pain. Pain. 1991;46:271-9.
- [Google Scholar]
- Outcome and complications of epidural analgesia in patients with chronic cancer pain. Cancer. 1998;83:2015-22.
- [Google Scholar]
- Cancer pain relief using chronic morphine infusion. Early experience with a programmable implanted drug pump. J Neurosurg. 1984;61:302-6.
- [Google Scholar]
- Technical complications during long-term subarachnoid or epidural administration of morphine in terminally ill cancer patients: A review of 140 cases. Reg Anesth. 1991;16:209-13.
- [Google Scholar]
- Long-term, open catheterization of the spinal subarachnoid space for continuous infusion of narcotic and bupivacaine in patients with “refractory” cancer pain. A technique of catheterization and its problems and complications. Clin J Pain. 1991;7:143-61.
- [Google Scholar]
- Epidural versus Intrathecal morphine-bupivacaine: Assessment of consecutive treatments in advanced cancer pain. J Pain Symptom Manage. 1990;5:18-26.
- [Google Scholar]
- Efficacy and technical complications of long-term continuous intraspinal infusions of opioid and/or bupivacaine in refractory nonmalignant pain: A comparison between the epidural and the intrathecal approach with externalized or implanted catheters and infusion pumps. In: Clin J Pain. Vol 14. 1998. p. :4-16.
- [Google Scholar]
- Long-term intrathecal infusion of morphine in the homecare of patients with advanced cancer. Acta Anaesthesiol. Scand. 1997;41:12-7.
- [Google Scholar]
- Long-term spinal analgesic delivery: A review of the preclinical and clinical literature. Reg Anesth Pain Med. 2000;25:117-57.
- [Google Scholar]
- Neuraxial medication delivery: The development and maturity of a concept for treating chronic pain of spinal origin. Spine (Phila Pa 1976). 2002;27:2593-605.
- [Google Scholar]
- Implantable Drug Delivery Systems Study Group. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: Impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040-9.
- [Google Scholar]
- Cost analysis of two implantable narcotic delivery systems. J Pain Symptom Manage. 1991;6:368-73.
- [Google Scholar]
- Comparative efficacy of epidural, subarachnoid, and intracerebroventricular opioids in patients with pain due to cancer. Cochrane Database Syst Rev. 2005;1:CD005178.
- [Google Scholar]
- Problems of long-term spinal opioid treatment in advanced cancer patients. Pain. 1999;79:1-13.
- [Google Scholar]
- Infection rates associated with epidural indwelling catheters for seven days or longer: Systematic review and meta-analysis. BMC Palliat Care. 2007;6:3.
- [Google Scholar]
- Evaluation of patients for implantable pain modalities: Medical and behavioral assessment. Clin J Pain. 2001;17:206-14.
- [Google Scholar]
- Long-term intraspinal infusions of opioids in the treatment of neuropathic pain. J Pain Symptom Manage. 1995;10:527-43.
- [Google Scholar]
- Epidural clonidine analgesia for intractable cancer pain. The epidural clonidine study group. Pain. 1995;61:391-9.
- [Google Scholar]
- Polyanalgesic Consensus Conference 2003: An update on the management of pain by intraspinal drug delivery--report of an expert panel. J Pain Symptom Manage. 2004;27:540-63.
- [Google Scholar]
- Polyanalgesic consensus conference 2007: Recommendations for the management of pain by intrathecal (intraspinal) drug delivery: Report of an interdisciplinary expert panel. Neuromodulation. 2007;10:300-28.
- [Google Scholar]
- Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: A meta-analysis of the literature. Spine J. 2008;8:488-97.
- [Google Scholar]
- Neurolytic celiac plexus block: A better alternative to opioid treatment in upper abdominal malignancies: An Indian experience. J Pain Palliat Care Pharmacother. 2005;19:15-20.
- [Google Scholar]
- Early ultrasound-guided neurolysis for pain management in gastrointestinal and pelvic malignancies: An observational study in a tertiary care center of urban India. Pain Pract. 2012;12:23-32.
- [Google Scholar]
- A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145-61.
- [Google Scholar]
- Current challenges in cancer pain management: Does the WHO ladder approach still have relevance? Expert Rev Anticancer Ther. 2007;7:1501-2.
- [Google Scholar]
- Interventional procedures for cancer pain management: Selecting the right procedure at the right time. J Support Oncol. 2010;8:60-1.
- [Google Scholar]
- Clinical trials in interventional pain management: Optimizing chances for success? Pain. 2010;151:571-4.
- [Google Scholar]






