Translate this page into:
Quality of Life and its Related Factors Among Iranian Patients with Metastatic Gastrointestinal Tract Cancer: A Cross-sectional Study
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Quality of life (QoL) is an important issue in all cancer patients; especially in patients with metastatic cancer. But there is very little information available about QoL in patients with metastatic gastrointestinal cancer.
Aims:
The aim of this study was to evaluate the quality of life and its associated factors among Iranian patients with metastatic gastrointestinal tract cancer.
Materials and Methods:
In this cross-sectional study, a total of 250 patients with metastatic gastrointestinal tract cancer were recruited from the one oncology center related to the Mazandaran University of Medical Sciences, Sari, between March 2012 and August 2013. Their QoL was evaluated using the EORTC QLQ-C30 questionnaire (Persian version).
Results:
In this study, the overall QoL score of patients with gastrointestinal tract cancer was 57.63, which was relatively optimal. There was a statistically significant relationship between symptoms scale and general health status domains of quality of life with age (P < 0.05). Also, there was a significant association between patients’ gender and their social functioning (P = 0.017) and also their emotional functioning (P = 0.015).
Conclusions:
The findings suggest that in patients with metastatic gastrointestinal cancers, the most affected functions in their QoL were social and emotional functioning which get worse with age. Thus, providing psychological counseling and psychotherapy services to deliver culturally appropriate mental health care and social support for these patients and their families’ which can lead to the improvement of QoL in these patients is strongly recommended.
Keywords
Gastrointestinal tract
Iran
Metastatic cancer
Quality of life
INTRODUCTION
Cancer is a major public health problem and one of the most common causes of death worldwide, including Iran.[12] About 50,000 new cases of cancer occur annually in the Iran.[3] Generally, gastrointestinal tract cancers, majority of which occurs in stomach, esophagus, colon, or rectum are most common organ system involved with more than 38% of all cancers and account for nearly half of all cancer causes of deaths in Iranian cancer patients.[34] Undoubtedly, being diagnosed of having a life threatening disease such as cancer is devastating and has an enormous effect on patients’ quality of life (QoL).[5] World Health Organization (WHO) defined QoL as the individuals’ perception of their position in life in the cultural context and in the value system in which they live, and in relation to their goals, expectations, standards, and concerns.[6] The measurement of QoL has become a vital and often required part of health outcomes appraisal. For patients with chronic disease such as cancer, which can affect the QoL of these patients and their families, measuring QoL provides a remarkable way to determine the impact of health care when cure is not possible.[78] The assessment of QoL can help to facilitate communication with cancer patients and identification of their individual preferences, for example, to select a specific treatment or care plan.[9] On the other hand, QoL is not only an important issue for individual cancer patients. It is also a matter of importance for policy makers and healthcare professionals. Therefore, the measurement of QOL is an important outcome measure in all cancer patients, but it becomes even more important in patients with metastatic cancers, where, with very few exceptions, cure is no longer the goal. In this situation, survival time prolongation, palliation of symptoms, and QoL optimization become the goals of care.[10] Previous studies have reported that in several different types of cancer including metastatic cancers, there was a relationship between the patients QoL scores and their clinical outcome.[11] QoL scores may be an independent predictor of survival in these patients.[1112] Despite the importance of assessing the QoL in cancer patients, especially in patients with metastatic cancer, and also the relatively high incidence rates of gastrointestinal tract cancers in Iran;[3413] little is known about QoL in patients with metastatic gastrointestinal cancer. The aim of this study was to evaluate the QoL with its various dimensions and also its associated factors such as demographics and clinical characteristics among Iranian patients with metastatic gastrointestinal tract cancer using a cancer-specific questionnaire, in Sari, northern Iran.
MATERIALS AND METHODS
In this cross-sectional study, after obtaining approval from the Mazandaran University of medical sciences ethical committee and informed consents from the patients, a total of 250 patients of both sexes, with metastatic gastrointestinal tract cancer referring to the one oncology center related to the Mazandaran University of Medical Sciences, Sari, Iran, for medical follow-up, were recruited. The study was performed between March 2012 and August 2013. Patients were eligible for the study if they had the ability to understand and the willingness to participate in the study and also had a histologically or cytologically confirmed diagnosis of metastatic gastrointestinal tract cancer and were informed of the diagnosis. Exclusion criteria were the presence of a known psychiatric or cognitive disorder or not willing to participate in the study. Patients who met the inclusion criteria were assessed regarding their QoL with a specific questionnaire by one researcher. The Persian versions of the European Organization for Research and Treatment of Cancer quality of life questionnaire-core 30 (EORTC QLQ-C30) and a background information form were used to assess patients’ QoL, sociodemographic data (gender, age, educational status, marital status, and employment status) and clinical characteristics. The EORTC QLQ-C30 is a cancer-specific, self-administered 30-question instrument for evaluating the QoL in cancer patients. The questionnaire items are grouped into five functional scales (physical functioning, role functioning, emotional functioning, cognitive functioning, and social functioning); three symptom scales (fatigue, nausea and vomiting, and pain); six symptom single-item scales (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties), and one global health status (GHS)/QoL scale. Of the 30 items, 28 are scored on four-point Likert scales and the remaining two items (29 and 30, for global health status) are scored on modified seven-point linear analog scales. Scores were derived from mutually exclusive sets of items, with scale scores ranging from 0 to 100 after linear transformation. Higher scores for the functioning and GHS/QoL scales indicated a higher level of functioning and a better QoL, respectively, whereas higher scores in symptom scales represented a higher level of symptom.[1114] The EORTC QLQ-C30 questionnaire has been previously used in cancer patients with good validity and reliability in Iran.[815] The questionnaire was completed by interview for illiterate or low-literacy patients.
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 16 (SPSS Inc., Chicago, IL). The Chi - square, Student's t-test, and Analysis of Variance (ANOVA) were used to analyze the data. Statistical significance was considered as P ≤ 0.05.
RESULTS
Out of 250 patients with metastatic gastrointestinal cancer, 146 (58.4%) were female and 104 (41.6%) were male.
The mean age of patients was 58.83 ± 8.02 years ranging from 44-70 and patients aged over 60 years accounted for 70% (n = 175). In the whole sample, 220 were married, 96 (38.4%) were illiterate, and 80 (32%) were unemployed. Among 250 patients, 193 (77.3%) had diabetes mellitus. In the whole sample, esophageal cancer was the most common gastrointestinal tract cancers. 216 (86.4%) of patients were under chemotherapy. The median time since start of treatment was 6 months in 152 (60.8%) of patients and only in 17 (6.8%) of patients the treatment took more than 25 months. The patients also resorted to religious therapies for treatment that the highest religious therapy used was through making a vow; where 130 ones (52%) were involved in 117 (46.8%) of patients abused psychoactive drugs and 44.4% (n = 111) of patients had a history of surgical treatment.
In 32% (n = 80) of patients, pain was their most important problem. Findings about QoL have shown that the mean scores for the functional scale, GHS, and symptoms scales in this study were 56.53, 59.63, and 39.08, respectively. (The score 100 indicates the best health condition and the score 0 implies the worst health condition). There was a significant association between patients’ gender and their social functioning (P = 0.017) and also their emotional functioning (P = 0.015). In functional scale of the QoL, the highest complaint from the patients was related to physical functioning with mean score 49.54 for men and the least cognitive functioning complaint was related to women with mean score 84.13 [Table 1].
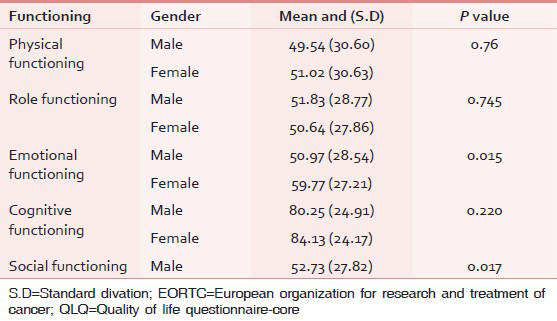
In the symptom scales, the highest complaint was of the financial difficulties with the mean score 89.95 for men and the least complaint was related to diarrhea with mean score 14.15 for women [Table 2].

Also a statistically significant relationship was found between the age and the organs of gastrointestinal tract which has been cancerous [Table 3].

Analyzing various domains of the patients’ QoL in terms of the age categories have been shown a statistically significant relationship between symptoms scale (P = 0.001) and global health status (P = 0.048) with age [Table 4]. There was no relationship between the QoL and sociodemographic variables such as age, gender, marital status, and employment status.

DISCUSSION
This study aims to analyze the QoL and its related factors in patients suffering from metastatic gastrointestinal cancer. In this research, the patients’ mean score has been 50.16 in physical function scale, 56.30 in social function, and 57.63 in general health status. In a study conducted by Blazeby et al., on the short-term outcomes of the patients undergoing esophageal cancer surgery and its relationship with QoL, the patients’ mean score was 79 in physical function, 69 in role playing function, 70 in emotional function, 80 in cognitive function, 72 in social function, and 62 in general health status.[16] Also, in the study by Kobayashi et al., the mean scores were as 77 for social function, 68 for role playing function, 67 for emotional function, 67 for cognitive function, 72 for social function, and 58 for general health status.[17] As observed, except for the cognitive function where the Iranian patients got higher score than similar studies, in the other domains, the score achieved by Iranian patients has been far lower than patients in other countries. In this study, the lowest score is related to the physical function while in the studies by Blazeby et al. and Kobayashi et al., the lowest score was related to general health status.[1617] The highest score concerning QoL was for cognitive function index that is consistent with the result of a study by Blazeby et al.[16] As seen from comparing QoL scores in Iran and the other countries, QoL in European countries is better than that in Iran. This issue can be attributed to the more supporting of these patients by ministry of health in those countries and also therapeutic supports given by cancer support services. However, in Iran due to financial problems and expensive treatment expenditures, the patients’ quality of life has been affected negatively. In this study, there was a significant statistical relationship between age and the disease, so that, the risk of developing gastrointestinal cancer increases with age (59 years old and more) which is consistent with the results of a studies by Blazeby et al. and Mahan et al.[1618] Moreover, there is a significant statistical relationship between age and disease symptoms index and also between age and general health status that is in line with results of the other.[192021] But Smith et al. and Redhwan et al. suggested that aging has resulted in quality life improvement.[2223] Perhaps the difference in the results is due to cultural differences and the number of research sample. In this study, there was no statistical relationship between type of metastatic gastrointestinal cancer and the patients’ QoL domains. The study by Schulz et al. indicated that the type of cancer has effect on the patients’ quality of life. For example, the individuals who are afflicted by rectum and colon cancer had lower life quality compared to those with breast cancer.[24] Besides, Viklun et al. asserted that the patients undergoing stomach cancer surgery have more optimal quality of life relative to the ones who had esophageal cancer surgery.[25] Maybe the reason behind the differences is the study instruments and its related dimensions, different culture, and samples number. In this study, all patients had gastrointestinal metastatic cancer and the same issue partially justifies the gap between the two studies. Also in this study, the rate of esophageal cancer in men was more than that in women. In various studies, the prevalence of esophageal cancer is higher in males than females, so that the results of these studies confirm the results of this study.[262728]
Regarding the relationship between marital status and OoL, the result of this study shows that there is no relationship between marital status and OoL. The result of the study by Ozaras et al. supports this finding.[29] However, the other studies’ results do not verify this finding.[303132] Perhaps, it can be claimed that the effect of marriage on the individuals’ OoL is due to the cultural and social differences. The result of the study implied that OoL has no relationship with job. The results of the studies by Taria et al., Engel et al., and Avis et al. revealed that job has relationship with QoL.[303233] However, the study findings by Ozaras et al. revealed no statistically significant relationship between job and QoL.[29] This gap could be attributed to difference in research time period, insurance system support in the country, and different study areas. The result of the study indicated that there is no significant statistical relationship between the level of education and QoL in the patients. The result of study that has been conducted by Kwan et al. is consistent with the result of this study in this regard.[34] However, the results of other studies found a significant relationship between the level of the education and QoL.[222932] These differences might be related to the different sample numbers, measurement instrument, and type or stage of the disease.
CONCLUSION
The study results have demonstrated that the individuals with metastatic gastrointestinal cancer were suffering from substantial disturbances in their QoL, especially in social function scale and emotional function which get worse with age. Thus, providing psychological counseling and psychotherapy services to deliver culturally appropriate mental health care and social support for these patients and their families’ which can lead to the improvement of QoL in these patients is strongly recommended.
The difference between the results of this study and similar studies in other countries is probably due to the lack of appropriate and sufficient supporting treatments, insufficient psychotherapy, counseling, and psychological services and no appropriate education program to the patients and their families. Some of the limitations of this study include that the patients who participate in this study did not have a good morale, although their participation in the study was good.
Also, this study was conducted in a single center in Mazandaran, Northern Iran. Therefore, we are not able to generalize this result to the whole population of patients with metastatic gastrointestinal tract cancer. Hence, the results of this study need to be interpreted with caution. Other studies in patients with various cancers to verify the generalizability of our findings are warranted.
ACKNOWLEDGMENTS
The financial support of Research Deputy of Mazandaran University of Medical Sciences is gratefully acknowledged. Also, the authors wish to thank all the study participants for their tremendous cooperation and support.
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212-36.
- [Google Scholar]
- Geographical spread of gastrointestinal tract cancer incidence in the Caspian Sea region of Iran: Spatial analysis of cancer registry data. BMC Cancer. 2008;8:137.
- [Google Scholar]
- Trends in incidence of gastrointestinal tract cancers in Western Iran, 1993-2007. Iran Red Crescent Med J. 2011;13:805-10.
- [Google Scholar]
- Does knowledge of cancer diagnosis affect quality of life? A methodological challenge. BMC Cancer. 2004;4:21.
- [Google Scholar]
- Psychol Med. 1998;28:551-8.
- The Quality of Life Scale (QOLS): Reliability, validity, and utilization. Health Qual Life Outcomes. 2003;1:60.
- [Google Scholar]
- Quality of life and its related factors among Iranian cervical cancer survivors. Iran Red Crescent Med J. 2013;15:320-3.
- [Google Scholar]
- How do nurses assess quality of life of cancer patients in oncology wards and palliative settings? Eur J Oncol Nurs. 2012;16:212-9.
- [Google Scholar]
- Quality of life in long-term survivors of metastatic breast cancer. Clin Breast Cancer. 2012;12:119-26.
- [Google Scholar]
- Quality of life analysis in patients with KRAS wild-type metastatic colorectal cancer treated first-line with cetuximab plus irinotecan, fluorouracil and leucovorin. Eur J Cancer. 2013;49:439-48.
- [Google Scholar]
- Quality of life data as prognostic indicators of survival in cancer patients: An overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7:102.
- [Google Scholar]
- Mortality trends of gastrointestinal cancers in Iranian population. Gastroenterol Hepatol Bed Bench. 2013;6(Suppl 1):S52-7.
- [Google Scholar]
- Quality of life after gastrectomy for cancer evaluated via the EORTC QLQ-C30 and QLQ-STO22 questionnaires: Surgical considerations from the analysis of 103 patients. Int J Surg. 2013;11(Suppl 1):S104-9.
- [Google Scholar]
- Factors affecting quality of life in cancer patients undergoing chemotherapy. Afr Health Sci. 2011;11:266-70.
- [Google Scholar]
- Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384-94.
- [Google Scholar]
- Effects of socioeconomic factors and cancer survivors’ worries on their quality of life (QOL) in Japan. Psychooncology. 2008;17:606-11.
- [Google Scholar]
- Quality of life in newly diagnosed patients with lung cancer in a developing country: Is it important? Eur J Cancer Care (Engl). 2006;15:293-8.
- [Google Scholar]
- Personality traits and psychosocial stress: Quality of life over 2 years following breast cancer diagnosis and psychological impact factors. Psychooncology. 2010;19:160-9.
- [Google Scholar]
- Factors associated with health-related quality-of-life in breast cancer survivors: Influence of the type of surgery. Jpn J Clin Oncol. 2009;39:491-6.
- [Google Scholar]
- Breast cancer in older women: Quality of life and psychosocial adjustment in the 15 months after diagnosis. J Clin Oncol. 2003;21:4027-33.
- [Google Scholar]
- Race/ethnicity, physical activity, and quality of life in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2009;18:656-63.
- [Google Scholar]
- Quality of life among women with breast cancer from University Kebangsaan Malaysia Medical Center, Malaysia. J Community Hlth. 2008;14:46-55.
- [Google Scholar]
- Predictors of quality of life in rural patients with cancer. Cancer Nurs. 2001;24:12-9.
- [Google Scholar]
- Quality of life and persisting symptoms after oesophageal cancer surgery. Eur J Cancer. 2006;42:1407-14.
- [Google Scholar]
- Psychometric properties of the Iranian interview-administered version of the World Health Organization's Quality of Life Questionnaire (WHOQOL-BREF): a population-based study. BMC Health Serv Res. 2008;8:61.
- [Google Scholar]
- The World Health Organization Quality of Life Instrument-short form (WHOQOL-BREF) in women with breast problems. Int J Clin Health Psychol. 2011;11:5-22.
- [Google Scholar]
- Quality of life and influencing factors in patients with a gynaecologic cancer diagnosis at Gazi University, Turkey. Asian Pac J Cancer Prev. 2010;11:1403-8.
- [Google Scholar]
- Associations among baseline variables, treatment-related factors and health-related quality of life 2 years after breast cancer surgery. Breast Cancer Res Treat. 2011;128:735-47.
- [Google Scholar]
- Quality of life of women with breast cancer: A qualitative study. Payesh. 2011;11:73-81.
- [Google Scholar]
- Predictors of quality of life in breast cancer patients. Acta Oncol. 2003;42:710-8.
- [Google Scholar]
- Quality of life among younger women with breast cancer. J Clin Oncol. 2005;23:3322-30.
- [Google Scholar]
- Quality of life among women recently diagnosed with invasive breast cancer: The pathways study. Breast Cancer Res Treat. 2010;123:507-24.
- [Google Scholar]






