Translate this page into:
Estimation and Comparison of Salivary Secretory Leukocyte Protease Inhibitor in Human Immunodeficiency Virus Patients and Healthy Individuals
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aim:
Transmission of human immunodeficiency virus (HIV) in the oral cavity is a rare event, despite detectable virus in saliva and oropharyngeal tissues of infected persons, unlike other mucosal sites. Secretory leukocyte protease inhibitor (SLPI) has been suggested as the main soluble factor responsible for the HIV inhibitory effect of saliva. The study was designed to estimate and compare the salivary SLPI levels in HIV patients and healthy controls. Furthermore, the relationship between salivary SLPI levels and disease severity was also investigated.
Materials and Methods:
Unstimulated whole saliva specimens were collected from 60 HIV-infected and 20 healthy subjects. Disease severity was determined by CD4 count in HIV subjects, who were divided into two groups: ≥200 cells/μL (n = 30) and < 200 cells/μL n = 30. Salivary SLPI levels were determined by enzyme-linked immunosorbent assay.
Results:
Numerically higher SLPI levels were observed in HIV subjects 193.342 ng/mL vs. 190.587 ng/mL; P = 0.517. A nonsignificant negative correlation was noted between CD4 counts and SLPI levels r = −0.037, P = 0.781.
Conclusion:
The salivary anti-HIV factor, SLPI, is not only preserved in HIV infection but its concentration may even get enhanced in the infection. However, the clinical significance of SLPI levels and disease severity should be investigated further with a larger sample of patients.
Keywords
CD4 count
Human immunodeficiency virus
Saliva
Secretory leukocyte protease inhibitor
INTRODUCTION
Despite the spread of knowledge about safer sexual practices to reduce the transmission of the human immunodeficiency virus HIV and the introduction of potent antiretroviral treatments, the pandemic produced by this virus continues to expand at an alarming pace. Acquired immunodeficiency syndrome is now the fourth cause of death worldwide.[1] For dental practitioners; in particular, the relative risk of HIV transmission through saliva from HIV seropositive persons is a subject of continuing concern. HIV-infected individuals frequently have mucosal and gingival lesions which can cause bleeding into the oral cavity, releasing virus into saliva and increasing the potential risk of transmission.[2] Epidemiological studies, however, suggest that the transmission of the HIV in the oral cavity is a rare event, despite detectable virus in saliva and oropharyngeal tissues of infected persons, unlike other mucosal sites. One reason for this apparent paradox is the presence of endogenous mucosal antiviral factors.[3]
Furthermore, it is well-established that human saliva inhibits HIV infectivity in vitro.[45678] It has been suggested that whole saliva, along with colostrum possess the highest levels of anti-HIV activity. Multiple studies have been conducted to identify the sources and identity of HIV inhibitory activity in the saliva of healthy and infected individuals.[9] Innate inhibitory molecules, such as virus-specific antibodies, mucins, thrombospondin, and soluble proteins, have been identified and partially characterized from saliva. An addition to the growing list is secretory leukocyte protease inhibitor SLPI, which potently protects adherent monocytes and activated peripheral blood mononuclear cells against HIV infection. In vitro studies have indicated that the levels of SLPI in saliva and semen, approximate levels required for HIV inhibition.[3] SLPI is an 11.7-kDa protein, which significantly blocks HIV infection at concentrations >100 ng/mL, which is naturally present in saliva, suggesting that this protein is likely a major anti-HIV component of oral secretions.[10]
Thus, we compared the salivary SLPI levels of HIV-infected patients with those of healthy controls. Furthermore among the study group, evaluations of salivary SLPI levels were done on the basis of: a) CD4 counts (≥ or < 200 cells/μL) and b) presence or absence of oral manifestations. This is the first study of its kind in Indian population exploring the role of salivary SLPI in HIV disease.
MATERIALS AND METHODS
Study group (Group I)
The patients visiting antiretroviral therapy (ART) clinic in Nagpur (India) were examined. A total of 60 HIV positive individuals, irrespective of sex, in the age group of 20-40 years were selected. None of the patients received ART. A thorough clinical examination, with special emphasis on oral examination was carried out. Diagnosis of oral lesions that were associated with HIV infection was made by using presumptive criteria given by EC-Clearinghouse, 1993.[11] CD4 count was estimated for the study group using BD FACS Caliber systemTN. These patients were divided into two subgroups, Group Ia with CD4 count < 200/μL (n = 30) and Group Ib with CD4 count ≥ 200/μL (n = 30).
Control group (Group II)
A total of 20 healthy individuals in the same age group were selected.
This study was approved by an institutional ethical committee and written consents were taken from each subject for their willingness to participate in the study.
Collection and processing of specimen
Whole, unstimulated saliva was collected in all the 80 subjects using spitting method. The samples were centrifuged at 2000 rpm for 5 min, and stored at −70°C. Quantitative detection of salivary SLPI was done, using Quantikine human SLPI Immunoassay kit by R and D Systems, USA.
Statistical analysis
SPSS (Statistical Package for Social Science) 14.0 software was used for the analysis. Independent samples t test (unpaired t test) was applied to see whether there was a significant difference in salivary SLPI levels in HIV +ve subjects and healthy controls; and among the patients with presence or absence of oral manifestations.
Analysis of variance was applied to see whether there was a significant difference in salivary SLPI levels among various groups, that is, low (<200) CD4 count group, high CD4 (≥200) group and the control group. Pearson's correlation test was applied to see if there was a significant correlation between the salivary SLPI levels and the CD4 cell counts in the HIV +ve subjects.
RESULTS
In total, 80 salivary specimens were collected for analysis. Mean age of the subjects in Group I was 31.28 years and in Group II was 29.40 years. Mean salivary SLPI level in Group I was 193.342 ng/mL (standard deviation, SD = 16.005), whereas in Group II it was 190.587 ng/mL (SD = 17.475). There was definitely an increasing trend in SLPI levels in saliva among HIV patients in comparison to levels in controls, but the trend did not reach a level of statistical significance (P = 0.517) [Table 1].
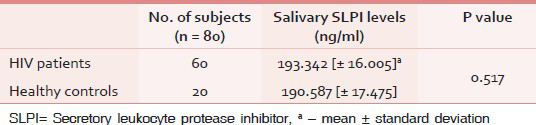
When the salivary SLPI levels were compared within the study population [Table 2], a nonsignificant (P = 0.798) increase in the value was noted in Group Ib (193.736 ng/mL). A nonsignificant higher SLPI levels were noted for either subgroups (Ia, Ib) as compared to Group II. A negative correlation was observed for salivary SLPI level and CD4 counts in Group I subjects (r = −0.037). However, this correlation was not statistically significant (P = 0.781).

On clinical examination, 44 of the 60 subjects (73.33%) in Group I were found to have at least one oral lesion. When the mean salivary SLPI levels were compared within the Group I, patients with oral manifestation showed a higher value (194.881 ng/mL) as compared to those without any oral lesion (189.108 ng/mL). However, this difference did not reach a level of statistical significance (P = 0.220).
DISCUSSION
For the dental professionals, the risk of HIV transmission through oral secretions is a topic of continuing concern, particularly in view of the growing number of infected people. Saliva can be passed from an HIV-infected individual to an uninfected person via sexual or nonsexual activities.[2] Interestingly, the frequency with which infectious HIV can be found in the saliva of HIV-infected patients is low, approximately 1%, although infectious virus can generally be isolated from the blood of untreated infected persons,[12] pointing toward the potential anti HIV activity of saliva. Thus, in its role as “gatekeeper” of the body, the oral cavity has evolved complementary strategies in its quest to protect the body against HIV. It has also been observed that whole saliva depleted of SLPI by elution from an affinity column containing anti-SLPI antibody exhibits a corresponding decrease in anti-HIV activity. Data suggest that SLPI may be one of the main factors responsible for the HIV inhibitory effect of saliva and is likely to be a major deterrent of HIV-1 transmission through oral secretions.[23]
The present study was conducted on 60 HIV seropositive patients and 20 healthy controls in the age group of 21-40 years. The upper age limit was selected as 40 years to exclude aging as a confounding factor, as both salivary SLPI concentration and the flow rate diminish with age.[13]
SLPI levels in the saliva samples from HIV patients were not significantly increased in comparison to those of uninfected controls in our study. These findings are consistent with results of previous studies,[31415] but in contrast with the results of some.[1617] Lin et al.,[16] suggested that increase in SLPI concentration in HIV patient was the result of decreased fluid secretion due to the disease. They also concluded that highly active antiretroviral therapy may enhance salivary SLPI concentration. In the present study, all the participants were newly diagnosed for HIV and none received antiretroviral treatment. Among the study group, decreased salivation was not a prominent oral manifestation and was noted only in one patient. Baqui et al.,[17] however, found equivalent levels of SLPI in the plasma of HIV infected as well as uninfected controls. They argued that increased transudation of the protein through the gingival sulcus contributed to higher saliva SLPI levels in the study group. Due to our study design, we could not evaluate the SLPI concentration in plasma. Future studies may be designed to estimate combined SLPI levels in saliva and plasma. There has been confusion regarding the exact reason for the increase in SLPI concentrations with HIV infection. Its mechanism of antiviral activity and whether its target resided at the level of the virus or the cell being infected has been a matter of debate.[616] Oral keratinocytes has been suggested as a potential source of the inhibitor which leads to natural abundance of SLPI in oral secretions. Exposure of oral epithelial cells to virus activates a signaling pathway that ultimately “turns on” SLPI expression. This kind of exposure can take place with receptive oral sex.[18] Oral sex, however, is still not prevalent in India and this difference in sexual practices can be one of the reasons why the SLPI levels were not significantly raised in our study. Additionally, elevation of protease inhibitor may arise as a local defense against inflammatory processes accompanying the disease, to minimize tissue damage.[16] However, the extent of gland inflammation was not determined in this study. Thus, the discrepancy may be explained by the difference in the stage of gland inflammation. However, even our result emphasizes the fact that SLPI is found in equivalent amounts in the saliva and salivary glands of both normal and HIV infected individuals. Similar findings have been reported.[56] This points to the potential importance of SLPI in thwarting the oral HIV transmission. This is further supported by the fact that only in the oral cavity does HIV gets exposed to SLPI. This is the reason why the virus is frequently detected in the tissues but infrequently in oral secretions.[35]
In the present study, mean SLPI concentration in unstimulated whole saliva of patients with low CD4 count (<200 cells/μL) was lower than that of patients with high CD4 count (≥200 cells/μL). However, the mean concentration of salivary SLPI of either group was higher than that of controls. A negative correlation (r = −0.037) for SLPI concentration in whole saliva and CD4 count was noted, but it did not reach statistical significance (P = 0.781). Presently, neither the biological nor the clinical significance of the association of SLPI with immune status is well understood at this time.[17] Hence, it cannot be predicted whether the decreased SLPI concentration in patients with low CD4 counts is indicative of a more advanced stage of HIV disease. Further studies with larger sample sizes are required to come to some kind of conclusion.
We also evaluated the salivary SLPI levels among group I subjects (with and without oral manifestations). However, the lesions were not categorized into erosive or nonerosive types. In order to evaluate the salivary SLPI concentration as influenced by the presence of oral lesions, it would be appropriate to specify the type of lesions, whether they were erosive or nonerosive or whether there was a periodontal breakdown.
Most commonly occurring oral lesion was oral candidiasis, followed by oral hairy leukoplakia. The immune status of the patients with oral manifestation was low as evidenced by their low CD4 count. As expected, there was definitely an increasing trend in SLPI levels in unstimulated whole saliva among HIV +ve patients with oral manifestations in comparison to levels in those without oral manifestations. But the increased trend of salivary SLPI between the two subgroups did not reach statistical significance (P = 0.220). A study reported comparable mean salivary SLPI levels between participants with and without current oropharyngeal candidiasis. However, SLPI levels were raised in patients with recent history of oropharyngeal candidiasis. They suggested that salivary SLPI levels are modified by candidal experience.[19] A possible biological explanation for this is that SLPI production gets upregulated in response to candidial infection in an attempt to inactivate the pathogenic microorganisms and resolve the clinical disease. Therefore, in patients who had already been exposed to candida organisms at the time of saliva collection, the salivary SLPI values may represent levels close to their individual thresholds. In our study, we did not enquire the subjects for the history of oral candidiasis. Thus, future studies should thoroughly record the recent history of oropharyngeal candidiasis, in addition to the current clinical status.
Our data may be reflective of our available HIV +ve population, and it should eventually be compared to trends in larger populations. The variation in SLPI levels may be caused by many factors like sample selection, sample size, study duration, saliva types (whole saliva in this study/glandular saliva), geographic and demographic variations, confounding factors (e.g., smoking) and gland inflammation. It is possible that other inflammatory conditions or microbial infections such as periodontal or HIV-associated oral pathogens could also trigger an increase in salivary SLPI. It has also been shown that cigarette smoking suppresses salivary SLPI levels.[19] When a bivariate analysis was performed keeping elevated protein level as dependent factor, there was no significant difference observed between cases and controls, with or without oral habits. This is indicative of the fact that in this study tobacco/alcohol habit was not a risk factor for change in protein level. However, the authors do believe that it might be a confounding factor and should be adjusted in similar studies performed. Therefore, future studies on salivary SLPI should take into account possible confounding influences by competing disease groups and local inflammatory conditions that may influence salivary SLPI production.
There was a nonsignificantly increased level of SLPI from whole, unstimulated saliva samples in HIV patients in comparison to uninfected controls. Although there were other trends noted in the data regarding a potential relationship between HIV patients with low CD4 counts and SLPI levels, the differences were not statistically significant compared to data for patients with high CD4 counts. Further studies with larger sample sizes are required to come to some kind of conclusion. SLPI is generally present at higher concentrations in tissues associated with lower rates of transmission of HIV.[6] Thus, if an inverse correlation exists between levels of SLPI and the risk or rate of HIV transmission, exogenously administered SLPI may afford protection against this lethal virus. Given its antimicrobial activity, SLPI may provide a valuable therapeutic option in the future treatment or prevention of infectious diseases. Establishing a role for SLPI in preventing oral HIV infection could lead to the development of novel interventions, such as using recombinant SLPI for HIV prevention.
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- Endogenous mucosal antiviral factors of the oral cavity. J Infect Dis. 1999;179(Suppl 3):S431-5.
- [Google Scholar]
- Human submandibular saliva inhibits human immunodeficiency virus type 1 infection by displacing envelope glycoprotein Gp120 from the virus. J Infect Dis. 1998;178:1635-41.
- [Google Scholar]
- The role of the oral environment in HIV-1 transmission. J Am Dent Assoc. 1998;129:851-8.
- [Google Scholar]
- Secretory leukocyte protease inhibitor: A human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. J Clin Invest. 1995;96:456-64.
- [Google Scholar]
- HIV in the oral cavity: Virus, viral inhibitory activity, and antiviral antibodies: A review. Crit Rev Oral Biol Med. 1993;4:461-6.
- [Google Scholar]
- Comparison of human immunodeficiency virus type 1-specific inhibitory activities in saliva and other human mucosal fluids. Clin Vaccine Immunol. 2006;13:1111-8.
- [Google Scholar]
- Secretory leukocyte protease inhibitor in vaginal fluids and perinatal human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;183:653-6.
- [Google Scholar]
- Classification and diagnostic criteria for oral lesions in HIV infection. EC-Clearinghouse on Oral Problems Related to HIV Infection and WHO Collaborating Centre on Oral Manifestations of the Immunodeficiency Virus. J Oral Pathol Med. 1993;22:289-91.
- [Google Scholar]
- Salivary concentration of secretory leukocyte protease inhibitor, an antimicrobial protein, is decreased with advanced age. Gerontology. 2001;47:246-53.
- [Google Scholar]
- Anatomic dissociation between HIV-1 and its endogenous inhibitor in mucosal tissues. Am J Pathol. 1997;150:1275-84.
- [Google Scholar]
- Salivary secretory leukocyte protease inhibitor is associated with reduced transmission of human immunodeficiency virus type 1 through breast milk. J Infect Dis. 2002;186:1173-6.
- [Google Scholar]
- Salivary secretory leukocyte protease inhibitor increases in HIV infection. J Oral Pathol Med. 2004;33:410-6.
- [Google Scholar]
- Enhanced secretory leukocyte protease inhibitor in human immunodeficiency virus type-1 infected patients. Clin Diagn Lab Immunol. 1999;6:808-11.
- [Google Scholar]
- Human immunodeficiency virus type 1 stimulates the expression and production of secretory leukocyte protease inhibitor (SLPI) in oral epithelial cells: A role for SLPI in innate mucosal immunity. J Virol. 2005;79:6432-40.
- [Google Scholar]
- Salivary secretory leukocyte protease inhibitor and oral candidiasis in human immunodeficiency virus type 1- infected persons. Infect Immun. 2004;72:1956-63.
- [Google Scholar]






